Covalent structures (1)

 4.3 Covalent structures (1) (3 hours)
4.3 Covalent structures (1) (3 hours)
This is the first part of sub-topic 4.3 Covalent structures. It includes Lewis structures, the 'octet rule, resonance hybrids and giant covalent structures. I have not included VSEPR theory to determine the shapes of simple molecules and ions on this page. Since the underlying theory is the same for 2, 3, 4 and 5 and 6 electrons pairs around the central atom I have prepared a separate page on VSEPR theory as 5 and 6 electron pairs are only covered at Higher Level in sub-topic 14.1. The total time for teaching all of sub-topic 4.3 is 5 hours.
Pause for thought
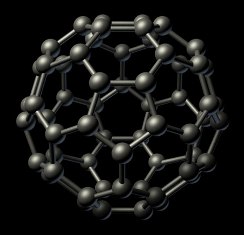 In 1996 the Nobel Prize for Chemistry was awarded to Robert F. Curl, Jr., Harry W. Kroto and Richard E. Smalley for their discovery of buckminsterfullerene. In the 1970s Harry Kroto from the University of Sussex was working on identifying molecules in interstellar gas clouds. Robert Curl and Richard Smalley were separately working at Rice University, Houston on using laser vaporisation techniques to produce clusters of atoms. In 1985 the two groups teamed up and by using a graphite target produced a molecule with a relative molar mass of 720 which corresponded to C60. By 1990 the structure of C60 had been confirmed by X-ray analysis. Buckminsterfullerene (named after the Buckminster Fuller Dome built for Expo '67 in Montreal, Canada) is a closed cage structure (see right) consisting of pentagons and hexagons. Each carbon atom is sp2 hybridized and bonded to three other carbon atoms. Other fullerenes , e.g. C70, have also been shown to exist. The discovery of fullerenes led to the whole new branch of science called nanotechnology which is covered in Option A: Materials. More information about the discovery of fullerenes can be found on the molecule of the month page published by Bristol University and also 'The discovery of fullerenes'
In 1996 the Nobel Prize for Chemistry was awarded to Robert F. Curl, Jr., Harry W. Kroto and Richard E. Smalley for their discovery of buckminsterfullerene. In the 1970s Harry Kroto from the University of Sussex was working on identifying molecules in interstellar gas clouds. Robert Curl and Richard Smalley were separately working at Rice University, Houston on using laser vaporisation techniques to produce clusters of atoms. In 1985 the two groups teamed up and by using a graphite target produced a molecule with a relative molar mass of 720 which corresponded to C60. By 1990 the structure of C60 had been confirmed by X-ray analysis. Buckminsterfullerene (named after the Buckminster Fuller Dome built for Expo '67 in Montreal, Canada) is a closed cage structure (see right) consisting of pentagons and hexagons. Each carbon atom is sp2 hybridized and bonded to three other carbon atoms. Other fullerenes , e.g. C70, have also been shown to exist. The discovery of fullerenes led to the whole new branch of science called nanotechnology which is covered in Option A: Materials. More information about the discovery of fullerenes can be found on the molecule of the month page published by Bristol University and also 'The discovery of fullerenes' ![]() produced by the American Chemical Society.
produced by the American Chemical Society.

It is worth contrasting this discovery and the subsequent award of the Nobel Prize with the work of Gilbert Lewis (1875-1946) in the first half of the last century. Much of the theory of covalent bonding underlying the whole of this topic was first proposed by Lewis and arguably he remains the most deserving chemist never to have won a Nobel Prize even though he was nominated several times. The politics behind the chemistry and how the Nobel Prize was awarded in the first part of the 20th century is covered well in the book Cathedrals of Science written by P. Coffey.
Nature of Science
Models are used by scientists as representations of the real world.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesDots, crosses, a dash (line) or any combination can be used to show electron pairs in Lewis (electron dot) structures. Coordinate covalent bonds (dative bonds) should be covered. Coverage of giant covalent structures should include silicon, silica, SiO2, and the allotropes of carbon (diamond, graphite, graphene, C60 buckminsterfullerene). International-mindedness(Nothing is listed in the programme for international-mindedness under this sub-topic) |
Teaching tipsA single covalent bond has been defined in sub-topic 4.2 as the sharing of a pair of electrons, one from each atom. This holds true for fluorine and chlorine as well as hydrogen. Then I show how the paradigm must gradually change to accommodate double and triple bonds (e.g. O2, N2, C2H4 etc.) then to accommodate both electrons coming from the same atom (CO, NH4+, Al2Cl6, H3O+ etc.) and then I go a bit further than the core to also include SO2 and SO3 and debate whether we have resonance hybrids or whether the 'octet' can be expanded and then finally SF6 where the 'octet' must be expanded. I also include electron deficient compounds such as beryllium dichloride, BeCl2 and boron trifluoride, BF3 where the 'octet' is not completed around the central atom. If I have good Standard Level students and certainly for Higher Level I then give them nitrogen(II) oxide, NO and they then see that the paradigm basically breaks down. This is explained fully in 'A modern paradigm'. Whether a molecule is polar or not depends not only on the relative electronegativities of its constituent atoms (covered in 4.2 Covalent bonding) but also its shape. Although shapes are part of this sub-topic (4.3) I have included shapes and molecular polarity in a separate page - Covalent structure (2). Finally a modelling set such as Molymod (or something similar) is a great way to illustrate the three-dimensional aspects of the structures of diamond, silicon, silicon dioxide (silica), graphite graphene and buckminsterfullerene. I think the hands-on experience is much better than a virtual 3-D tour on a flat 2-D screen but the first video in the 'other resources' below does give a good animation of this. | Study guide
Pages 26 and 28 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Covalent structures (1). For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Covalent structures (1) questions. Vocabulary listLewis structure IM, TOK, 'Utilization' etc.See separate page which covers all of Topic 4 & 14. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A video showing the different allotropes of carbon. Worth watching, not so much for the commentary, but for the excellent animation.
2. Another useful video on the allotropes of carbon produced by Dr Ainissa Ramirez of Yale University.
3. The structure and bonding in silicon and silicon dioxide are also important and are covered in this video by Richard Thornley - an IB teacher from the United Nations International School in New York.
4. A good podcast by the Royal Society of Chemistry on

 IB Docs (2) Team
IB Docs (2) Team 





























