Reduction reactions
 20.1 Types of organic reactions (7 hours)
20.1 Types of organic reactions (7 hours)
4. Reduction reactions (Estimated 1 hour)
Pause for thought
Both sodium borohydride and lithium aluminium hydride are white inorganic solids but students are not very likely to have 'hands on' experience of either of them in a school laboratory. Sodium borohydride can be used in the presence of a protic solvent such as water or ethanol so is relatively safe to use but if you do use lithium aluminium hydride practically in your school then it is wise to be alert to the potential risks associated with it. It can react violently with water and even reacts with the water vapour in the air.
LiAlH4(s) + 4H2O(l) → LiOH(aq) + Al(OH)3(s) + 4H2(g)

 In 2011 it was reported that scraping an old sample of lithium aluminium hydride with a spatula caused a flash fire in a university laboratory (see above) – probably because it reacted with moisture in the air to form hydrogen gas. This potential hazard that friction can cause a fire is listed under the properties of lithium aluminium hydride on PubChem.
In 2011 it was reported that scraping an old sample of lithium aluminium hydride with a spatula caused a flash fire in a university laboratory (see above) – probably because it reacted with moisture in the air to form hydrogen gas. This potential hazard that friction can cause a fire is listed under the properties of lithium aluminium hydride on PubChem.
Considering that the new programme is so keen on using modern terminology as stated in the IUPAC Gold Book elsewhere on the syllabus (e.g. carboxamide rather than amide, phenylamine rather than aniline etc.) it seems slightly strange that the IB still refers to two of the most important reducing agents used in organic chemistry, sodium borohydride and lithium aluminium hydride, by their old names. The preferred IUPAC names for sodium borohydride, NaBH4, and lithium aluminium hydride, LiAlH4, are sodium tetrahydridoborate(III) and lithium tetrahydridoaluminate(III) respectively.
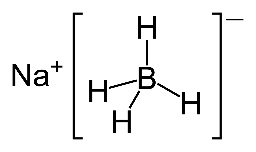
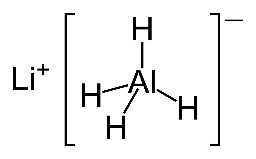
Both can be considered to be a source of hydrogen anions, H−, and they reduce carbonyl compounds by quite complex nucleophilic addition reactions.
Generally lithium aluminium hydride is a more powerful reducing agent than sodium borohydride so is preferentially used to reduce carboxylic acids to primary alcohols via the aldehyde intermediate whereas either can be used to reduce aldehydes or ketones to their respective alcohols. When carrying out these reduction processes with lithium aluminium hydide it is important to keep water out of the first step and ethoxyethane (diethylether), C2H5OC2H5, is often used as an aprotic solvent. This is dried beforehand using sodium metal which reacts with any water present and is then removed before the solvent is required. Once the intermediate has formed, acid, or sometimes an alcohol, is added to obtain the product.
One question a good student might ask is why the reduction of nitrobenzene is included in this sub-topic. This appears to be an isolated reaction which does not easily relate to any other chemistry on the syllabus except for the fact that the formation of nitrobenzene is given as an example of electrophilic substitution. The reaction is important as the product, aniline (and its analogues), is the precursor for the aniline dye industry (see Mauve: How One Man Invented a Colour That Changed the World in Books for enjoyment ). Aniline is also used in the production of polyurethanes and paracetamol is synthesised by reacting 4-aminophenol with ethanoic anhydride. However, as none of the reactions of aniline appears anywhere in the core. AHL or any of the options, the inclusion of the reduction of nitrobenzene seems to be just demanding the recall of factual information with no real purpose. I have given tin and concentrated hydrochloric acid as the reducing agent as it can be carried out practically in a school laboratory. It also fulfils the syllabus requirement of a two stage conversion but in fact nitrobenzene can also be reduced directly to phenylamine using hydrogen gas and a palladium or nickel catalyst at 200-300 oC.
Nature of Science
Organic reactions fall into a number of different categories. By understanding different types of organic reactions and their mechanisms, it is possible to synthesize new compounds with novel properties for use in diverse applications.
Collaboration between scientists on investigating the synthesis of organic compounds using new green chemistry pathways involves ethical and environmental implications.
Learning outcomesAfter studying this sub-topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesAlthough typical conditions and reagents for all reactions should be known (e.g. catalysts, reducing agents, reflux etc.); more precise details, such as specific temperatures, do not need to be included.International-mindednessHow important in the global context is organic chemistry to green and sustainable chemistry? |
Teaching tipsThis is a rather factual topic as students do not need to understand how the reducing agents work. Their use really comes with the next sub-topic as they provide a method of selectively reducing various compounds to produce different classes of compounds so are useful in synthesis reactions. Students should know that the use of sodium borohydride or lithium aluminium hydride involves a two-step reaction and that water is normally kept out of the first step. Since the mechanism is not required the NaBH4 or LiAlH4 can be written over the arrow rather than as the reactant in a chemical equation, i.e.
You will also need to cover the reduction of nitrobenzene to phenylamine. There are various ways in which this reduction can be brought about and the syllabus does not specify any particular one. One classic method is to first reflux the nitrobenzene with mixture of tin metal and concentrated hydrochloric acid. The tin acts as the reducing agent and forms the phenylammonium ion, C6H5NH3+. C6H5NO2 + 7H+ + 6e− → C6H5NH3+ + 2H2O Then sodium hydroxide solution is added to release the free amine. C6H5NH3+ + OH− → C6H5NH2 + H2O | Study guide
Page 92 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Reduction reactions. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Reduction reactions questions. Vocabulary listsodium borohydride, NaBH4 IM, TOK, Utilization etc.See separate page which covers all of Topics 10 & 20. |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. An interesting video which goes beyond the syllabus requirements to explain why lithium aluminium hydride is a stronger reducing agent than sodium borohydride. However students should be able to follow much of it as it is based upon the relative electronegativities of boron and aluminium.
![]() Why LiAlH4 is a stronger reducing agent than NaBH4
Why LiAlH4 is a stronger reducing agent than NaBH4
2. A video showing practically how nitrobenzene can be converted into phenylamine. The reducing agent used is iron rather than tin but the principle is similar.
![]() Reduction of nitrobenzene to phenylamine
Reduction of nitrobenzene to phenylamine

 IB Docs (2) Team
IB Docs (2) Team 












