2 &12. Atomic structure (1)
 Atomic structure (1)
Atomic structure (1)
For each question choose the answer you consider to be the best.
1. Which is the correct definition of the atomic number of an atom?
A. The total number of neutrons and protons in the nucleus of an atom
B. The total number of neutrons, protons and electrons in an atom
C. The number of electrons in the outer energy level of an atom
D. The number of protons in the nucleus of an atom
2. Which statement about isotopes is correct?
A. Isotopes have the same molecular formula but a different structural formula.
B. Isotopes have the same number of neutrons in atoms of the element
C. Isotopes of atoms of the same element have the same electron configuration.
D. Isotopes of atoms of the same element have the same mass number.
3. An atom of thorium has a mass number of 234. Which is correct concerning the atomic number for this isotope and the number of neutrons in its nucleus?
4. The two isotopic abundances of element Z are 90% of 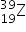 and 10% of
and 10% of 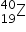 . What is the relative atomic mass of Z?
. What is the relative atomic mass of Z?
A. 39.10
B. 39.90
C. 39.50
D. 19
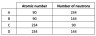
5. The table shows the number of protons, neutrons and electrons in five different species.

Which two species are isotopes of the same element?
A. P and Q
B. Q and R
C. P and R
D. S and T
6. How many neutrons are there in the ion 32P3- ?
A. 15
B. 17
C. 18
D. 32
7. What are valence electrons?
A. The total number of electrons in an ion
B. The number of electrons that need to be removed to form a positive ion
C. The number of electrons that need to be added to form a negative ion
D. The number of electrons in the highest energy level of the atom
8. The first six successive ionization energies of an element (in kJ mol-1) are 577, 1820, 2740, 11600, 14800 and 18400. Which group in the periodic table does the element belong to?
A. 1
B. 2
C. 13
D. 15
9. What are the correct units for Plank's constant (h) ?
A. J s
B. J s–1
C. J
D. J m–1
10. Which statement is correct about a line emission spectrum?
A. Electrons release energy as they move from a higher level to a lower level
B. Electrons absorb energy as they move from a higher level to a lower level
C. Electrons release energy as they move from a lower level to a higher level
D. Electrons absorb energy as they move from a lower level to a higher level
11. Which are correct for the hydrogen emission spectrum?
I. The spectrum consists of lines converging at higher energy.
II. The lines in the UV region are due to electronic transitions to the lowest
energy level.
III. The lines are due to electrons absorbing energy as they move from
higher energy levels to lower energy levels.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
12. The electronic configuration of potassium is [Ar]4s1. How many occupied main energy levels are there in an atom of potassium?
A. 1
B. 4
C. 6
D. 19
13. A transition metal ion, M3+ has the electron configuration [Ar]3d5. What is the atomic number of the transition metal?
A. 23
B. 25
C. 26
D. 28
14. What is the total number of electrons in p orbitals in a bromide ion, Br−?
A. 5
B. 6
C. 9
D. 18
15. Which species contains only one unpaired electron?
A. Fe2+
B. Sc3+
C. Cu2+
D. Cr3+
16. Which is correct about the element antimony, Sb?

17. Which are correct statements about orbitals?
I. The plane of px orbitals is at 90o to the planes of py and pz orbitals.
II. p orbitals can form new hybrid orbitals with s orbitals
III. A p orbital can contain a maximum of six electrons
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
18. Which equation represents the fourth ionization energy of a metal M?
A. M4+(g) → M5+(g) + e−
B. M3+(s) → M4+(s) + e−
C. M(g) → M4+(g) + 4e−
D. M3+(g) → M4+(g) + e−
19. Which is a correct statement about ionization energies?
A. First ionization energies generally decrease across a period.
B. The first ionization energy of potassium is higher than the first ionization energy of sodium.
C. The second ionization energy of lithium is higher than the second ionization energy of beryllium.
D. The second ionization energy of magnesium is lower than the first ionization energy of magnesium.
20. What is the electronic configuration of an atom with Z = 52?
A. 1s22s22p63s23p64s23d104p65s24d105p4
B. [Kr]5s25d105p4
C. [Kr]5s24d104p4
D. 1s22s22p63s23p64s23d104p65s25p105d4
 Answers
Answers
1. D, 2. C, 3. B , 4. A, 5. D, 6. B, 7. D, 8. C 9. A, 10. A,
11. A, 12. B 13. C, 14. D, 15. C, 16. B., 17. A, 18. D, 19. C, 20. A.
Download the Atomic structure ![]() Topics 2 & 12 MC (1) test
Topics 2 & 12 MC (1) test ![]()
Download the answer grid with the correct answers to cut out for Topics 2 & 12 MC (1) ![]()

 IB Docs (2) Team
IB Docs (2) Team