Acid base calculations
 18.2 Calculations involving acids and bases (4 hours)
18.2 Calculations involving acids and bases (4 hours)
Pause for thought
How weak is a weak acid?
The IB does not require students to be able to solve quadratic equations when it comes to acids and bases calculations. This means that they are required to make an assumption that the equilibrium concentration of a weak acid in solution is the same as the concentration of the undissociated acid. How valid is this assumption?
Consider a 0.100 mol dm-3 aqueous solution of ethanoic acid (Ka = 1.8 x 10-5)
CH3COOH(aq) ⇄ CH3COO–(aq) + H+(aq)
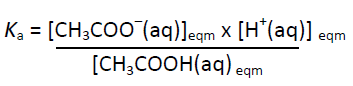
Since water is only very slightly dissociated (Kw = 1.00 x 10-14 at 298 K)
[CH3COO–(aq)] = [H+(aq)] and [CH3COOH(aq)]eqm = (0.100 – [H+(aq)])
[H+(aq)]2 = 1.8 x 10-5 x (0.100 – [H+(aq)])
[H+(aq)]2 + (1.8 x 10-5 x [H+(aq)]) – 1.8 x 10-6 = 0
Solving the quadratic equation gives [H+(aq)] = 1.33 x 10-3 mol dm−3 which gives a pH value of 2.9
If the approximation is made that the equilibrium concentration of the acid is the same as the undissociated acid then
[H+(aq)]2 = 1.8 x 10-5 x 0.100 = 1.8 x 10-6 mol2 dm−6
[H+(aq)] = (1.8 x 10-6)½ = 1.34 x 10-3 mol dm−3 which gives a pH value of 2.9
Clearly the difference is quite small so the approximation is valid.
However consider a weak solution of a stronger acid e.g. 1.00 x 10−3 mol dm−3 dichloroethanoic acid.
CHCl2COOH(aq) ⇄ CHCl2COO–(aq) + H+(aq)
From Section 21 of the data booklet, pKa of dichloroethanoic acid = 1.35; so Ka = 10−1.35 = 4.5 x 10-2
Without the approximation:
[H+(aq)]2 + 4.5 x 10-2[H+(aq)] – 4.5 x 10-5 = 0
which solves to give [H+(aq)] = 9.79 x 10-4 mol dm−3 (pH value of 3.0)
Using the approximation:
[H+(aq)] = (4.5 x 10−2 x 1.00 x 10−3)½ = 6.7 x 10-3 mol dm−3 (pH value of 2.2)
Now there is clearly a large difference and the approximation is not valid. Students need to understand that the approximation only really works for relatively concentrated solutions of relatively weak acids. Ostwald’s dilution law is beyond the syllabus but generally the acid should be less than 5% dissociated for the approximation to be valid.
Nature of Science
By applying the equilibrium law the strengths of acids and bases can be determined and related to their molecular structure thus providing evidence for scientific theories.
| Clarification notesThe value of Kw is temperature dependent.
International-mindednessThis topic is mathematical in nature. Although chemists around the world speak many different languages, the fact that mathematics is a universal language enables them to communicate more objectively. |
Teaching tipsThis sub-topic very much builds on the core sub-topic 8.3 pH scale but goes much further. Make sure that your students fully understand how to manipulate logarithms to the base ten. I start by writing the equation and the equilibrium expression for the dissociation of pure water at 25 oC so that they understand that [H+(aq)] = [OH−(aq)] and then apply the definition of pH. They should know that the dissociation of water is endothermic (since the reverse reaction of neutralisation is exothermic) so can deduce that Kw increases as the temperature increases. Show them the graph of Kw against temperature and get them to work out [H+(aq)], [OH-(aq)], pH and pOH for pure water at various temperatures. I then revise (US review) how to calculate the pH of strong acids and bases and reinforce the fact that pH depends on the hydrogen ion concentration not on the volume of solution present. From the equations for weak acids and bases with water they can deduce the equilibrium expressions and work out the pH (or pOH) by assuming that the acid (or base) is only very slightly dissociated which avoids having to solve a quadratic equation. For a good class you can calculate the pH without making the assumption (see above) and show that the result is very little different. Get them to understand the meaning of pKa and pKb and give them examples of different acids and bases together with their dissociation constants so that they can put them in order of increasing acidity (or basicity). | Study guide
Pages 64 - 66 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Calculations involving acids & bases. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Acid-base calculations questions. Vocabulary listIonic product of water, Kw IM, TOK, Utilization etc.See separate page which covers all of Topics 8 & 18 |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A pdf file on acid base calculations ![]() from Stephen K. Lower at Simon Fraser University goes a little bit further than the IB requires but is a good source of background information.
from Stephen K. Lower at Simon Fraser University goes a little bit further than the IB requires but is a good source of background information.
2. There are many videos (of varying quality) on acid base calculations. Richard Thornley, a teacher at the International School of Genoa, has made some especially for the IB. This shows calculations involving how Kw varies with temperature.
3. Ranking acids and bases in order of strength according to their Ka, Kb, pKa and pKb values also by Richard Thornley.

 IB Docs (2) Team
IB Docs (2) Team 















