Measuring energy changes

 5.1 Measuring energy changes (4 hours)
5.1 Measuring energy changes (4 hours)

All fossil fuels provide their energy through an exothermic reaction involving combusting the fuel in air.
Pause for thought
1. Problems with enthalpy diagrams
It is not difficult to find enthalpy level diagrams in books or on the Internet and most teachers will draw out simple ones themselves. The typical diagram below which actually gives the values for two specific reactions, one exothermic and one endothermic is from the website avogadro.co.uk.
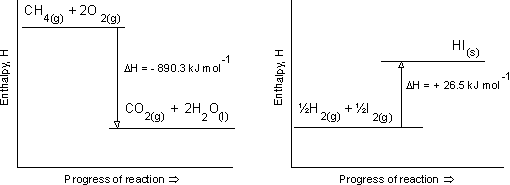
What is perhaps not appreciated is the labelling of the y-axis. It is an enthalpy diagram so it should be labelled enthalpy as above - the problem is that no scale can ever be given as the enthalpy values for both the reactants and the products can never be known. This important fact is not obvious from these diagrams and there is a danger that they can give a misleading message to students if this fact is not made clear. All that can ever be measured is the difference between the two values, i.e. the enthalpy change, ΔH. The x-axis also causes some problems. 'Progress of reaction' is often used when the activation energy or some intermediate is also shown on the diagram. All that a simple enthalpy level diagram shows is the initial and final states and they could quite legitimately be placed one above the other so that no x-axis is required (as we do for energy diagrams of energy levels within an atom).
2. What is the 'true' value?
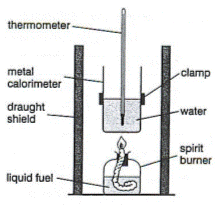 One of the best practicals for students to do is to determine the enthalpy of combustion of a flammable liquid using a spirit burner underneath a beaker containing a known mass of water (see right). The experiment is fraught with problems such as incomplete combustion, heat loss and mass loss through evaporation when finding the mass of the burner before and after the combustion. However the calculations involved in determining the result cover this sub-topic well and the experiment also lends itself to a thorough and meaningful evaluation and a discussion as to how the experimental method could be improved. To do this thoroughly the literature ('accepted') value needs to be known so that the percentage error can be determined. The problem is "What is the literature value?"
One of the best practicals for students to do is to determine the enthalpy of combustion of a flammable liquid using a spirit burner underneath a beaker containing a known mass of water (see right). The experiment is fraught with problems such as incomplete combustion, heat loss and mass loss through evaporation when finding the mass of the burner before and after the combustion. However the calculations involved in determining the result cover this sub-topic well and the experiment also lends itself to a thorough and meaningful evaluation and a discussion as to how the experimental method could be improved. To do this thoroughly the literature ('accepted') value needs to be known so that the percentage error can be determined. The problem is "What is the literature value?"
The IB seems to place great emphasis on students stating their experimental value together with the total uncertainties. Yet the various data books available never state an uncertainty in their values. One liquid that could be used in the spirit burner is methylbenzene (toluene). The following values can be found in the standard data books for the enthalpy of combustion of methylbenzene.
Literature values for the enthalpy of combustion, ΔHc⦵, of methylbenzene (left)1
1. – 3908 kJ mol-1
2. – 3909 kJ mol-1
3. – 3907 kJ mol-1
4. – 3910 kJ mol-1
This emphasises several points. Firstly, that students should give the source of their 'literature value' as different sources give different values and secondly why is the IB so 'hung up' on students at this age calculating all their uncertainties when in the scientific world the recognized data books do not include them? It also strongly elicits the TOK question "Which one is the true value?"
It might also be worth pointing out that some Internet sources give other different values. For example, 5. – 3906 kJ mol-1 and 6. – 3916 kJ mol-1.
1Sources:
1. Handbook of Chemistry and Physics, 72nd Ed. 1992
2. Chemistry Data Book ,Stark and Wallace, 2nd Ed. 1982 and IB Chemistry Data Booklet (pre-2009 version)
3. Binas, 1992
4. IB Chemistry Data Booklet (post 2009 version)
5. J.Chem.Eng.Data, 1969 14 (1), p 102-106 (Note this actually gives the value as -934.49 ± 0.12 kilocalories mol-1 which is equivalent to -3906 ± 0.5 kJ mol-1 so it does include uncertainties.)
6. Wikipedia (Note this gives the value as – 40.589 MJ kg-1 equivalent to 3740 kJ mol-1. However this is the Low Heating Value which means the enthalpy of vaporization of water has been subtracted. Adding this value for 4 mols of water gives an extra – 176 kJ of energy which brings the total to – 3916 kJ mol-1.
Nature of Science
The conservation of energy is an example of one of the fundamental principles of science.
Measuring energy transfers between systems and surroundings requires making careful observations.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesEnthalpy changes of formation, ΔHf⦵, and combustion, ΔHc⦵, should be covered. Combustion reactions and reactions in aqueous solution should be covered. Temperature is not a part of the definition of standard state, but 298 K is commonly used. Standard state refers to the normal, most pure stable state of a substance measured at 100 kPa. It can be assumed that the density and specific heat capacities of aqueous solutions are equal to those of water, although students should be aware of this assumption. The specific heat capacity of water is given in Section 2 of the data booklet. The heat capacity of the calorimeter and heat losses to the environment need to be considered. International-mindednessThe SI unit of temperature is the Kelvin (K). The USA uses the Fahrenheit scale (°F) for all non-scientific communication but almost all other countries use the Celsius scale (°C), which has the same incremental scaling as the Kelvin scale. |
Teaching tipsInitially I introduce the topic by getting students to understand that all chemical reactions involve bond breaking and bond formation to emphasise that energetics is a key concept underlying the whole of Chemistry. Chemists cannot ever state the exact potential energy of the reactants and products but they can measure the energy change as products are formed from reactants. It does seem quite obvious that energy is required to break a bond but it is not so intuitively obvious that the same quantity of energy is released when the bond is formed. The difference between energy and enthalpy is quite difficult at this level and it will probably suffice to just say that enthalpy equates to heat energy. Technically ΔH refers to the enthalpy change at constant pressure so it actually includes the internal energy of the system minus any work done by the system when it expands (or plus the work done on the system when the volume contracts). I have included a question on in this the accompanying questions but do stress that it is beyond the syllabus. This sub-topic is a gift for practical work and students can be given several good practicals to illustrate the theory. Clearly the key to carrying out most enthalpy calculations is knowing exactly what is meant by specific heat capacity. Students should not just learn the expression q = mcΔT they should understand what is actually happening when the temperature of a substance increases (or decreases). If you stress the units of specific heat capacity, kJ kg-1 K-1, then they do not have to memorize the equation. They can also see that it is not only the mass of the liquid that is changing. If the metal container or stirrer is being heated up then the energy change will involve other values for specific heat capacities and the concept of the heat capacity of the calorimeter can be introduced. | Study guide
Pages 38 & 39 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Measuring energy changes. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Measuring energy changes questions. Vocabulary list:endothermic IM, TOK, 'Utilization' etc.See separate page which covers all of Topic 5. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A nice demonstration by Professor Bob Burk of Carleton University, Ottowa of an endothermic reaction between two solids, barium hydroxide and ammonium nitrate. It also illustrates the importance of entropy change too.
2. One for you to try at home - or perhaps not? An exothermic reaction in the palm of your hand.
3. Students know that their own calorimetry experiments give poor results due mainly to heat loss and incomplete combustion. This video shows how a bomb calorimeter works to overcome these problems and determine accurate enthalpies of combustion. (Remember though that a bomb calorimeter is not on the syllabus.)

 IB Docs (2) Team
IB Docs (2) Team 



















