Energy cycles
 15.1 Energy cycles (3 hours)
15.1 Energy cycles (3 hours)
Pause for thought
The idea of using an energy cycle to solve problems involving Hess's Law has already been introduced in sub-topic 5.2 Hess's Law. This Higher Level sub-topic involves the use of energy cycles that contain the lattice enthalpy and or enthalpies of hydration and solution. A Born-Haber cycle is simply a special case of Hess’s Law. It refers to the enthalpy change of formation of an ionic compound. It can either proceed directly or in a series of separate steps which involve several different enthalpies changes which assume that the salt formed is 100% ionic. Most of these enthalpy changes can be measured experimentally so the purpose of the Born-Haber cycle is either to find an unknown enthalpy value or to compare the experimental or theoretical value with that determined by the cycle. For the IB this usually means determining the value for the lattice enthalpy. If there is good agreement between the experimental and theoretical lattice enthalpy which has been calculated assuming a purely ionic structure then it is reasonable to assume the compound is close to being 100% ionic. However if the two values do not agree then it suggests that the salt contains some degree of covalent character. There were problems with this on the last programme as the theoretical values in the then data booklet had already taken into account any covalent character. The current data booklet for first exams in 2016 does not contain the theoretical values and in the guidance notes it states that the polarising effect of some ions which produces covalent character in some essentially ionic substances will not be assessed.
A Born-Haber cycle should be exactly that - a cycle. Many text books and websites actually give it as an enthalpy level diagram. If we take sodium chloride as the classic example then the basic reaction is the reaction of one mole of solid sodium metal with half a mole of chlorine gas to give one mole of solid sodium chloride. This can either proceed directly or via the gaseous atoms and ions as shown below:
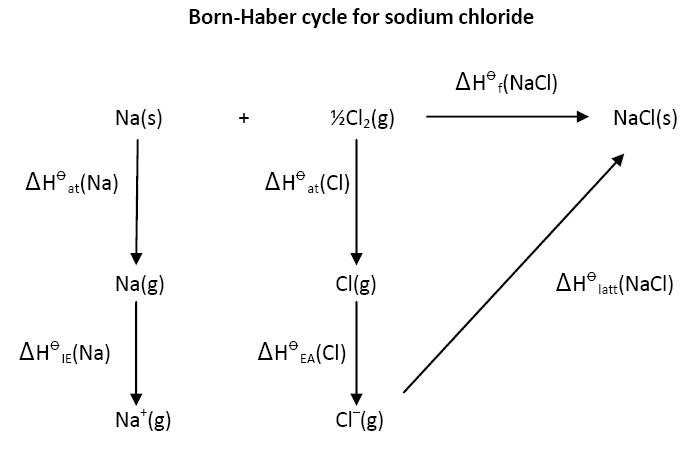
The great strength of the cycle is that is does not matter whether the values are positive or negative (or their quantitative value). It is clear that
∆H![]() f(NaCl) = ∆H
f(NaCl) = ∆H![]() at(Na) + ∆H
at(Na) + ∆H![]() at(Cl) + ∆H
at(Cl) + ∆H![]() IE(Na) + ∆H
IE(Na) + ∆H![]() EA(Cl) + ∆H
EA(Cl) + ∆H![]() latt(NaCl)
latt(NaCl)
Contrast this with the enthalpy diagrams (below) for sodium chloride and calcium oxide. Students need to know both the size and the signs of the values of the enthalpy changes (which in the case of electron affinities can be different) before they can draw the diagram correctly. To me the relationship between all the values also seems less obvious. The IB of course accepts either cycles or enthalpy diagrams as correct.
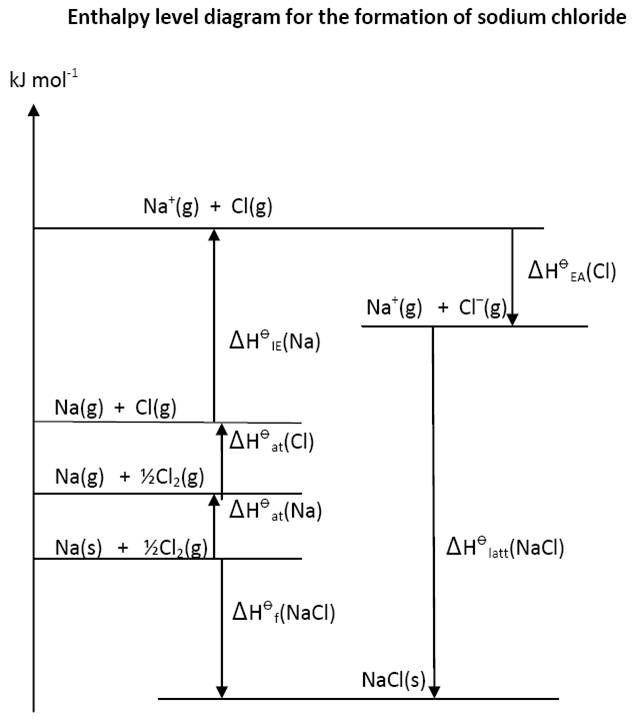
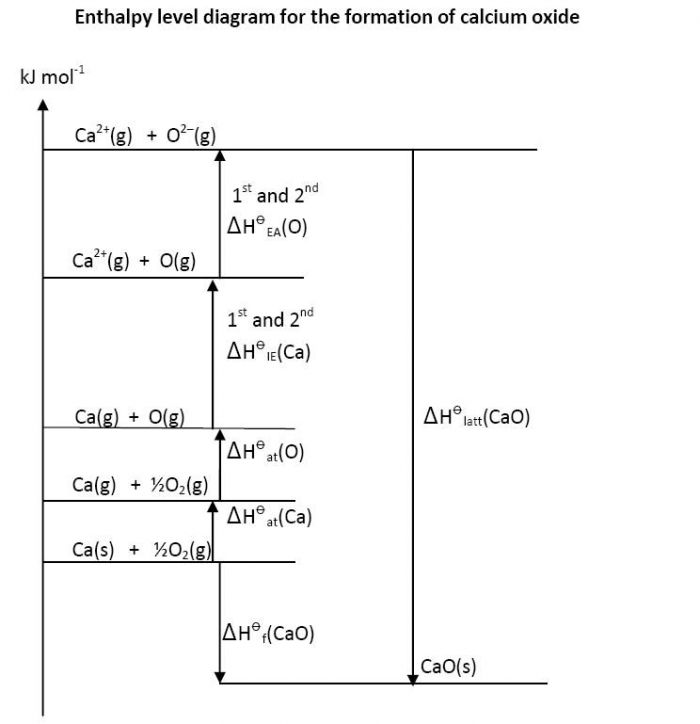
Nature of Science
Energy cycles permit the calculation of values that cannot be determined directly. This relates to the importance of replicating quantitative measurements to ensure reliability.
Learning outcomesAfter studying this topic students should be able to: Understand
Apply their knowledge to:
| Clarification notesThe polarizing effect of some ions which produces covalent character in some essentially ionic substances will not be assessed. International-mindednessThe significance of being able to obtain indirect measurements using energy cycles for things that cannot be measured directly is of global importance. For example, snow depth cover, borehole temperatures, |
Teaching tipsIt is worth introducing the underlying concepts when you cover Ionic bonding & structure without actually specifying that you are doing a Born-Haber cycle. I then start this topic by asking students to write the equation for the formation of sodium chloride from its elements. I then ask them to build up the cycle definition by definition so they should be able to deduce that first the solid sodium needs to be turned into gaseous sodium and the chlorine to chlorine bond needs to be broken. Once the gaseous atoms have been obtained the sodium is ionized by losing an electron and the chlorine is ionized by gaining an electron. They should be able to tell you whether each process is exothermic or endothermic. Applying Hess’s law it is then easy to see that the most exothermic value must be for the formation of solid sodium chloride from its gaseous ions. Note that lattice enthalpy can be either exothermic (when the solid salt is formed from its gaseous ions) or endothermic (when the salt is broken down into its gaseous ions) depending upon which way round it is defined. You can then ask them to draw Born-Haber cycles for other ionic compounds such as magnesium chloride and magnesium oxide. They should realise that for the magnesium they will need the first and second ionization energies. Similarly for oxygen they need the first and second electron affinities and the overall process is endothermic. They will see that the values for the lattice energies are much higher which leads into a discussion on the factors affecting lattice enthalpies. Even though arguably it is not now on the syllabus I still get them to understand that the Born-Haber cycle assumes that the solid formed is 100% ionic. This holds true for salts such as sodium chloride but not for silver iodide where there is considerable covalent character. I approach hydration energies by asking why sodium chloride has a high melting point (801 oC) and yet the temperature only falls by about 1 oC when it is dissolved in water - so where does the energy to break down the lattice in solution come from? Once you have explained this enthalpy cycle and students realise why all sodium salts are soluble you can use a similar cycle to explain that lithium is above potassium in the reactivity series because of its high hydration energy. | Study guide
Page 42 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Energy cycles. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Energy cycles questions. Vocabulary listBorn-Haber cycle
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A straightforward video showing how a Born-Haber cycle for magnesium chloride can be used to find the value for the electron affinity of chlorine.
2. Lattice enthalpy and electron affinity specifically for the IB by Richard Thornley.
3. Factors (radii, charge and charge density) affecting the size of the lattice enthalpy - also by Richard Thornley.

 IB Docs (2) Team
IB Docs (2) Team 























