Strong & weak acids & bases

 8.4 Strong and weak acids and bases (1 hour)
8.4 Strong and weak acids and bases (1 hour)
Pause for thought
This sub-topic is really a very basic introduction to the differences between strong and weak as applied to acids and bases. Where it can get slightly tricky is when students are asked to determine the relative strengths of acids and bases from experimental data. This data might be conductivity measurements or more likely the extent of dissociation in water. Standard Level students should understand about acid (and base) dissociation constants as equilibrium constants are covered in the previous topic on equilibrium. but they are not expected to know specifically about pKa and pKb. It would not be unreasonable to give Standard Level students the values of Ka for two different weak acids and ask them which is the strongest.
The problem is that Section 21 in the IB data booklet is specifically referred to in the 'Guidance' section of this sub-topic 8.4 (see below). This Section lists the values for the strengths of common acids and bases, but it only gives them as pKa or pKb values even though pKa and pKb are not on the Standard Level programme.
Some examples are given below:
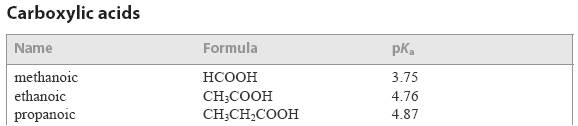

Either you will need to explain to Standard Level students how pKa relates to Ka (and how pKb relates to Kb) or you will need to convert the pKa and pKb values to Ka and Kb yourself to give to students or find them in other data sources.
Nature of science
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe terms dissociation and ionization are interchangeable. A list of weak acids and bases can be found in Section 21 of the data booklet. International-mindednessNothing is listed on the syllabus under this heading. |
Teaching tipsThis is a relatively straightforward topic to teach as students really only need to grasp the fact that a strong acid is completely dissociated into its ions in water whereas a weak acid is only slightly dissociated. It is then obvious that a strong acid will be a better conductor of electricity than a weak acid with the same concentration. Weak and strong bases follow a similar logic. For the equation for their reaction with water students must now include water on the reactant side so that OH–(aq) can be shown as a product, i.e. NH3(aq) + H2O(l) ⇄ NH4+(aq) + OH–(aq) Students will need to understand that a strong acid readily loses a proton so its conjugate base will be weak as it will not easily regain the proton. A similar logic can be applied to strong bases which have weak conjugate acids. It can be useful to give them water and alcohol as an example. Water is actually slightly more dissociated than ethanol even though both are extremely weak. This means that the ethoxide ion will be an even stronger base than the hydroxide ion. Finally, make sure that students understand that when it comes to a titration the same volume of a particular concentration of sodium hydroxide will be required to react with equal volumes of equimolar solutions of either a strong or a weak acid. This is so even though the hydrogen ion concentration in solution will be less for the weak acid. This of course is because more acid will dissociate as the H+(aq) ions are neutralised in order to restore the position of equilibrium but students often do not realise this. What will be different is the amount of heat evolved. | Study guide
Page 61 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Strong & weak acids & bases. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Strong & weak acid & bases questions. Vocabulary listStrong acid / strong base IM, TOK, Utilization etc.See separate page which covers all of Topic 8. Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. Richard Thornley's laser gun explains the concept of strong and weak when applied to acids and bases.
Strong & weak acids & bases (1) ![]()
2. Jonathan Bergmann and Aaron Sams interactively talk about strong & weak acids & bases.
Strong & weak acids & bases (2) ![]()

 IB Docs (2) Team
IB Docs (2) Team 















