Proteins & enzymes

 B.2 Proteins & enzymes (4 hours)
B.2 Proteins & enzymes (4 hours)
Pause for thought
This topic requires students to understand the primary, secondary, tertiary and quaternary structures of proteins. They can easily understand how the primary structure is obtained by hydrolysing the protein and then analysing the amino acid sequence. However how can the 3-D structure of proteins be obtained unequivocally? ⦵
In 1962 Max Perutz was awarded the Nobel prize for the determining the structure of haemoglobin, the first 3-D structure of any protein to be elucidated. The prize was also awarded to John Kendrew, who worked closely with Perutz, and who later determined the structure of another protein, myoglobin, which carries oxygen to muscles.
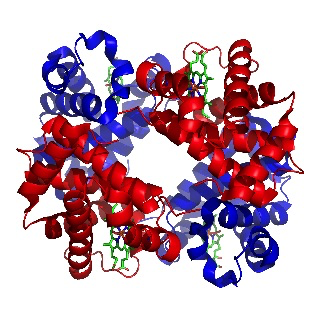
Image of haemoglobin by Richard Wheeler
(Released under the GNU Free Documentation License)
Max Perutz had been working on the problem using X-rays since 1937. He was able to obtain good X-ray images but the problem was interpreting them. It was finally solved by using the technique of isomorphous replacement. This involved introducing a heavy metal atom such as mercury into the haemoglobin, which subtly altered the X-ray pattern. By analysing these alterations the first 3-D structure of a protein was deduced in 1959.
Since then the structures of many different proteins have been determined and are held on a data base called the Protein Data Bank. Modern methods of determining protein structures involve X-ray crystallography, NMR spectroscopy, and electron microscopy with each having its strengths and weaknesses. In fact, even now it is still very difficult to be absolutely certain about whether a proposed structure is actually correct. In a nice Nature of Science statement the PDB itself says,
“When looking at PDB entries, it is always good to be a bit critical. Keep in mind that the structures in the PDB archive are determined using a balanced mixture of experimental observation and knowledge-based modelling. It often pays to take a little extra time to confirm for yourself that the experimental evidence for a particular structure supports the model as represented and the scientific conclusions based on the model.”
Nature of Science
The conclusion that DNA, and not protein (as originally thought), carries the information for inheritance is an example of collaboration and peer review achieved after experimental work on different continents.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe names and structural formulas of the amino acids can be found in Section 33 of the data booklet. Refer to alpha helix and beta pleated sheet structures, and to fibrous and globular proteins giving examples of each. The use of Rf values and locating agents in paper chromatography should be covered. Km and Vmax are not required when teaching enzyme kinetics (they are covered at HL in B.7). International-mindednessThe Universal Protein Resource (UniProt), a consortium of bioinformatics institutes, acts as a resource for the scientific community. It provides high-quality, comprehensive, and freely accessible data on protein sequence and functional information. |
Teaching tipsIt is worth starting this topic by looking at the acid properties of carboxylic acids and the basic properties of amines then discussing the structures of 2-amino acids which contain both functional groups. Use Section 33 to show all the important 2-amino acids. You will need to talk about their structures in low and high pH solutions and the formation of zwitterions. Get students to determine the structures of the dimers and trimers that can be formed when they condense together (see previous sub-topic). Note that two different amino acids can combine to give two different dipeptides and three different amino acids used once each can combine to give six different trimers. Get students to predict the types of interactions that can occur between different amino acid residues in proteins. These include covalent bonding (peptide bond), ionic bonding, hydrogen bonding, disulfide bridges, dipole-dipole interactions and London dispersal forces. You will need to get students to sketch graphs of how enzymes lower the activation energy of a biological reaction, how the rate of reaction reaches a plateau once all the active sites on the substrate are occupied and also how enzyme function varies with pH and temperature. To analyse proteins stress that they must first be hydrolysed to break them down into their constituent individual 2-amino acids. The analysis then depends upon their different polarities (chromatography) or their different isoelectric points (electrophoresis). | Study guide:
Pages 125 - 127 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Proteins & enzymes. For short-answer questions see Proteins & enzymes questions together with the worked answers on a separate page Proteins & enzymes answers. Vocabulary list2-amino acid Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. Two very short but succinct videos giving visual representations of the the primary, secondary, tertiary and quarternary structures of proteins. You could ask your students to compare the two videos and state what else they could contain to make the chemistry more complete.
2. A search for proteins on the Internet produces some interesting 'health conscious' links. One that has some information that might be useful is by Dr Friedli ( a yoga expert!)
3. How to separate proteins experimentally using polyacrylamide gel electrophoresis (PAGE).
4. A good video from the RCSB Protein Data Bank on how enzymes work.

 IB Docs (2) Team
IB Docs (2) Team 

































