Paper 2
Essential information
- The SL examination tests the core part of the programme and the HL examination tests the core and the Additional Higher Level (AHL) material. The split of core to AHL on the HL paper is approximately 60% to 40%.
- The examination follows Paper 1 (after a short break) on the same day (afternoon).
- The SL exam lasts for 1 hour 15 minutes. It consists of short answer questions and extended response questions. All questions are compulsory, i.e. unlike the Paper 2 in past programmes, there is no choice of questions.
- The maximum mark for the SL paper is 50 marks and it accounts for 40% of the overall mark for chemistry.
- The HL exam lasts for 2 hour 15 minutes. Like the SL paper, it consists of short answer questions and extended response questions. All questions are compulsory.
- The maximum mark for the HL paper is 90 marks and it counts for 36% of the overall mark for chemistry.
- A calculator (programmable/graphic display calculators are permitted) is required and a clean copy of the IB Chemistry Data Booklet is supplied. A simple translation dictionary can be used by non-native English (or French or Spanish) speakers.
- The examination tests 50% objectives 1 and 2 and 50% objective 3.
- The number of marks available for each sub-question is given.
- There is a five minute reading time before the examination starts.
- All questions are in the form of commands using the command terms listed in the IB Chemistry Subject Guide.
- Some of the questions will test understanding of the nature of science in context.
- The amount of marks for each topic will approximately be related to the number of recommended teaching hours for each topic.

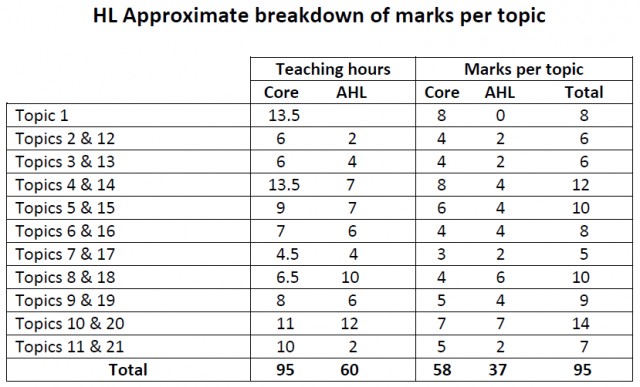
Note that from May 2019 the marks per topic have been adjusted slightly so that the total is now 90 rather than 95.
- All the answers must be recorded in the boxes provided (see example below) on the examination paper. If more space is required then additional answer sheets should be attached and a note made in the box that the answer continues elsewhere. This is because the exam is e-marked and both the person scanning and the examiner need to be aware that your answer is continued outside the box otherwise it may not get seen.

An example of an e-marking box
- Until May 2011 candidates could be penalised by one mark on the whole paper if they omitted or used the wrong units for an answer and could also be penalised by one mark on the whole paper if they gave an answer to the incorrect number of significant figures. Since the introduction of e-marking this is no longer the case. Candidates are not penalised for errors in units or significant figures, unless it is specifically referred to in the markscheme and only one mark can be lost for the wrong use of significant figures on the whole paper. Units are normally no longer a problem as the units are detailed in the question e.g. "Determine the standard entropy change, in J K−1 for the decomposition of dinitrogen monoxide" (M19HLP2TZ2Qu2(g)(i)) or "Calculate the concentration of NaHCO3, in mol dm−3" (M19HLP2TZ2Qu5(d)(iii)).
- Candidates must show their working for answers involving calculations. Part of the reason for this is because examiners apply 'error carried forward' (ECF) so that the same error is not penalised twice (or more times).
- At the end of the examination the extra sheets should be attached and the number of extra sheets recorded on the front cover.

 IB Docs (2) Team
IB Docs (2) Team