Misuse of formulas
Students often use mathematical formulas to solve chemical problems in exams and sometimes arrive at the wrong answer. This page looks at why this is so and encourages students to work always from first principles rather than mindlessly 'plug' values into a formula that they are unable to derive for themselves. It gives worked examples and provides some further practice questions with worked answers.
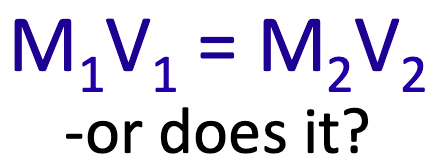
Introduction
Both mathematics and science make much use of formulas to solve problems. There is nothing inherently wrong in using a formula. In fact in many cases it can save a considerable amount of time. For example, Einstein is reputed to have said that compound interest is the eighth wonder of the world – “He who understands it earns it… he who doesn’t… pays it.”. A person who 30 years ago put $1000 dollars in an investment account that paid 7% interest per year ($0.07 per dollar) would have gained 30 x $70 in interest if the interest had been withdrawn at the end of each year giving a total sum of $3100 if the original investment is also included. However if the interest had been left in the account the formula A = P (1 + r)t (where A = the future value, P = the principal investment amount, r = the interest rate and t = time) shows that the $1000 initial investment would have grown to $7612 after 30 years. If it had been left for 50 years the figures would have been $4500 and $29460 respectively. Clearly using this particular formula saves all the tedious work of working out the increase and compounding it for each year etc.
Unfortunately some chemistry students who make considerable use of formulas in exams often arrive at the wrong answer because they have either misused a formula or have been unable to apply the correct formula to solve the problem.
There are several reasons why this may be so.
Reasons for errors using formulas
1. The formula is remembered wrongly.
A simple formula often used to find the concentration of a solution is:
c = n / V (where c is the concentration, n is ‘number of moles’ (actually amount in mol) and V is the volume).
I’ve seen several variations of this in exams, e.g. c = V / n or n = c / V. The key to stating formulas correctly is to check the units. Concentration is normally expressed as mol dm−3. Once a student realises this the formula is redundant as mol dm−3 literally means the amount in mol divided by the volume in litres (dm3). Another example where the formula is often quoted wrongly is the relationship between the velocity of light (c), the frequency (ν) and the wavelength (λ).
Looking at the units it is obvious that velocity (m s−1) = wavelength (m) x frequency (s−1), i.e. c = λν.
2. The formula is applied wrongly
One of the most common examples of this is the formula M1V1 = M2V2 which students often use to solve titration problems. It does work in the simplest case when one mole of an acid is neutralised by one mole of a base,
e.g. HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
but certainly does not work when it comes to the neutralisation of diprotic acids,
e.g. H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l).
My advice is to never use it! Instead always work from first principles.
Worked example:
26.80 cm3 of a solution of sulfuric acid was required to neutralise 25.00 cm3 of 0.150 mol dm−3 potassium hydroxide solution. Calculate the concentration of the sulfuric acid solution in mol dm-3.
Step 1: Write the stoichiometric equation
H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l)
Step 2: Calculate the amount (in mol) of potassium hydroxide present.
1000 cm3 of 0.150 mol dm−3 KOH contains 0.150 mol
so 25.00 cm3 contains (25.00/1000) x 0.150 = 3.75 x 10−3 mol.
Step 3: Calculate the amount (in mol) of sulfuric acid required to neutralise the potassium hydroxide solution.
From the equation 1 mol of H2SO4 neutralises 2 mol of KOH so amount of H2SO4 required = ½ x 3.75 x 10−3 = 1.875 x 10−3 mol.
Step 4: Calculate the concentration of the sulfuric acid in mol dm−3.
26.80 cm3 of H2SO4 contains 1.875 x 10−3 mol
1000 cm3 of H2SO4 contains (1000/26.80) x 1.875 x 10−3 = 0.0700 mol
so the concentration of the sulfuric acid = 0.0700 mol dm−3 (to 3 significant figures).
3. A formula cannot be used at all.
A back to basics stepwise approach is the only way to answer titration questions where a solid, rather than a solution, is being titrated as the formula M1V1 = M2V2 cannot be applied.
Example:
What mass of calcium carbonate will react exactly with 20.00 cm3 of 0.200 mol dm−3 hydrochloric acid?
Equation: 2HCl(aq) + CaCO3(s) → CaCl2(aq) + CO2(g) + H2O(l)
Amount of acid reacting = 20.00/1000 x 0.200 = 4.00 x 10−3 mol
Since two mol of HCl react with one mol of CaCO3
Amount of CaCO3 that reacts = ½ x 4.00 x 10-3 = 2.00 x 10−3 mol
M(CaCO3) = 40.08 + 12.01 + (3 x 16.00) = 100.09 g mol−1
Mass of CaCO3 that reacts with the acid = 100.09 x 2.00 x 10−3 = 0.200 g ( to 3 significant figures).
4.The wrong units are used.
The classic example of where this often occurs is when students use the equation
ΔG = ΔH – TΔS
ΔG and ΔH values are usually quoted in kJ mol−1 but entropy values are normally quoted in J K−1 mol−1. Students constantly need reminding that they must divide the entropy value by 1000 to convert it into kJ K−1 mol−1 when using it in the equation. Of course they also need to ensure the temperature is given in Kelvin not oC.
Other examples of formulas where units must be consistent are the gas equation pV = nRT and rate equations as the units of the rate constant depend upon the overall order of the reaction.
5. Problems with the Henderson–Hasselbalch equation.
This formula causes particular problems although strictly speaking calculations involving buffer solutions should only appear in Paper 3.
The theory of buffer solutions is covered at Higher Level in the AHL Topic 18 but buffer calculations only appear in two of the options in Paper 3. However even though buffers are not coved at Standard Level in the core, Option D (see D.4 pH regulation of the stomach) includes the application of the Henderson–Hasselbalch equation at both SL and HL whereas in option B (B.7 Proteins and enzymes) it is only on the HL syllabus. The use of the Henderson–Hasselbalch equation in Option D can cause real problems for Standard Level students who have not covered either equilibrium calculations or the definitions of Ka, Kb, pKa and pKb.
The Henderson–Hasselbalch equation is given in Section 1 of the IB data booklet but it seems unwise for students to use it if they do not fully understand it. It is easy to use wrongly and and I have never taught it to my students as I prefer them to be able to work out all buffer calculations from first principles.
Example using first principles
Calculate the pH of a buffer solution made by adding 20.0 cm3 of 1.00 mol dm−3 sodium hydroxide solution to 40.0 cm3 of 1.00 mol dm-3 ethanoic acid solution. Section 21 of the IB data booklet gives the pKa of ethanoic acid = 4.76
(Standard level students need to understand that Ka is the equilibrium constant (acid dissociation constant) for a weak acid and that pKa = − log10Ka (this is rather similar to pH = − log10[H+(aq)]). The relationship Ka = 10−pKa is also helpful as the data booklet only lists pKa values, not Ka values.)
Worked solution:
Step 1: Write the equation for the reaction.
CH3COOH(aq) + NaOH(aq) → CH3COONa(aq) + H2O(l)
After the reaction all the sodium hydroxide solution will have been neutralised to form sodium ethanoate and the excess ethanoic acid remains.
Step 2: Calculate the amounts of the sodium ethanoate and the excess ethanoic acid in the resulting solution.
Initial amount of NaOH = 20.0/1000 x 1.00 = 2.00 x 10−2 mol
Initial amount of CH3COOH = 40.0/1000 x 1.00 = 4.00 x 10−2 mol
Since all the NaOH reacts to form CH3COONa
Final amount of CH3COONa = 2.00 x 10−2 mol
Final amount of CH3COOH = 4.00 x 10−2 − 2.00 x 10−2 = 2.00 x 10−2 mol.
Step 3: Calculate the concentrations of the sodium ethanoate and the excess ethanoic acid in the resulting solution.
Final volume = 20.0 + 40.0 = 60.0 cm3 (assuming the volumes are exactly additive).
[CH3COONa(aq)] = 1000 / 60.0 x 2.00 x 10−2 = 0.333 mol dm−3
[CH3COOH(aq)] = 1000 / 60.0 x 2.00 x 10−2 = 0.333 mol dm−3
Step 4: Write the equilibrium expression for the dissociation of ethanoic acid.
CH3COOH(aq) ⇌ CH3COO−(aq) + H+(aq)
Ka = [H+(aq)] x [CH3COO−(aq)] / [CH3COOH(aq)]
Step 5: Substitute the values for the concentration of the acid and the salt into the equilibrium expression to find [H+(aq)].
Ka = [H+(aq)] x [CH3COO−(aq)] / [CH3COOH(aq)] = [H+(aq)] x 0.333 / 0.333 = [H+(aq)]
Ka = 10−4.7 so
[H+(aq)] = 10−4.76 mol dm−3
Step 6: Convert the hydrogen ion concentration into its pH value
pH = − log10[H+(aq)]
so pH = − log1010−4.76 = 4.76
Note that this example shows that at the half-equivalence point (i.e. when exactly half of the excess weak acid has been neutralised by the base, so that [acid] = [salt]) the pH = pKa.
Practice questions
Answer the following questions from first principles (i.e. without using any mathematical formulas). The worked answers can be accessed by clicking on the ‘hidden’ symbol.
1. Calculate the concentration of an aqueous solution of sodium nitrate containing 1.70 g of sodium nitrate dissolved in 30.0 cm3 of the solution.
M(NaNO3) = 22.99 + 14.01 + (3 x 16.00) = 85.00 g mol−1
Amount of NaNO3 in 30.00 cm3 = 1.70 ÷ 85.00 = 0.0200 mol
Amount of NaNO3 in 1000 cm3 (1 dm3) = 1000 / 30.00 x 0.0200 = 0.667 mol
Concentration of NaNO3(aq) = 0.667 mol dm−3.
2. Three separate solutions, 40.0 cm3 of 2.00 mol dm−3 HCl(aq), 50.0 cm3 of 1.60 mol dm-3 HCl(aq) and 30.0 cm3 of 1.8 mol dm−3 HCl(aq) are combined to make one solution. What is the concentration of this new solution?
Amount of HCl in 40.0 cm3 of 2.00 mol dm−3 HCl(aq) = (40.0 / 1000) x 2.00 = 8.00 x 10−2 mol.
Amount of HCl in 50.0 cm3 of 1.60 mol dm−3 HCl(aq) = (50.0 / 1000) x 1.60 = 8.00 x 10−2 mol.
Amount of HCl in 30.0 cm3 of 1.8 mol dm−3 HCl(aq) = (30.0 / 1000) x 1.80 = 5.40 x 10−2 mol.
Total amount of HCl present = (8.00 + 8.00 + 5.40) x 10−2 = 0.214 mol
Total volume = 40.0 + 50.0 + 30.0 = 120 cm3
Concentration = 0.214 x 1000/120 = 1.78 mol dm−3.
3. What volume of 0.250 mol dm−3 sulfuric acid is required to neutralise exactly 15.0 cm3 of 0.150 mol dm−3 barium hydroxide solution?
H2SO4(aq) + Ba(OH)2 → BaSO4(aq) + 2H2O(l)
Amount of Ba(OH)2 in 15.0 cm3 = (15.0 / 1000) x 0.150 = 2.25 x 10−3 mol
1 mol of H2SO4 reacts with 1 mol of Ba(OH)2
Amount of H2SO4 required = 2.25 x 10−3 mol
Volume of 0.250 mol dm−3 H2SO4 required = (1000 / 0.250) x 2.25 x 10−3 = 9.00 cm3.
4. What volume of carbon dioxide measured at STP will be evolved when 2.10 g of sodium hydrogen carbonate, NaHCO3 is reacted with excess hydrochloric acid?
NaHCO3(s) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l)
M(NaHCO3) = 22.99 + 1.01 + 12.01 + (3 x 16.00) = 84.01 g mol−1
Amount of NaHCO3 = 2.10 ÷ 84.01 = 0.0250 mol
1 mol of NaHCO3 reacts to produce 1 mol of CO2
Amount of CO2 evolved = 0.0250 mol
1 mol of any gas occupies 22.7 dm3 at STP (from Section 2 of the IB data booklet)
Volume of CO2 evolved = 0.568 dm3 = 568 cm3
5. (a) What will be the pH of the buffer solution prepared by adding 25.00 cm3 of 1.25 mol dm−3 NaOH(aq) to 60.00 cm3 of 1.20 mol dm−3 CH3COOH(aq). (Ka of CH3COOH = 1.74 x 10−5 at 298 K)
CH3COOH(aq) + NaOH(aq) → CH3COONa(aq) + H2O(l)
Initial amount of NaOH = 25.0/1000 x 1.25 = 3.125 x 10−2 mol
Initial amount of CH3COOH = 60.0/1000 x 1.20 = 7.200 x 10−2 mol
Since all the NaOH reacts to form CH3COONa
Final amount of CH3COONa = 3.125 x 10−2 mol
Final amount of CH3COOH = 7.200 x 10−2 − 3.125 x 10−2 = 4.075 x 10−2 mol
Final volume = 25.0 + 60.0 = 85.0 cm3 (assuming the volumes are exactly additive).
[CH3COONa(aq)] = 1000 / 85.0 x 3.125 x 10−2 = 0.3676 mol dm−3
[CH3COOH(aq)] = 1000 / 85.0 x 4.075 x 10−2 = 0.4794 mol dm−3
Ka = [H+(aq)] x [CH3COO−(aq)] / [CH3COOH(aq)] = [H+(aq)] x 0.3676 / 0.4794 = 0.767 x [H+(aq)]
Ka of CH3COOH = 1.74 x 10−5
[H+(aq)] = 1.74 x 10−5 / 0.767 = 2.27 x 10−5
pH = − log10[H+(aq)] = 4.64
5.(b) The same buffer solution can be prepared by dissolving solid sodium ethanoate in a solution of ethanoic acid. What mass of sodium ethanoate would need to be dissolved in 50.0 cm3 of 1.20 mol dm−3 CH3COOH(aq) to give a buffer solution with a pH of 4.64? Assume that when the salt is dissolved it does not affect the volume of the solution.
CH3COOH(aq) ⇌ CH3COO−(aq) + H+(aq)
Ka = [H+(aq)] x [CH3COO−(aq)] / [CH3COOH(aq)]
CH3COOH(aq) = 1.20 mol dm−3 and since the pH must be 4.64, [H+(aq)] = 10−4.64 and the value of Ka is given in 5.(a).
Ka = 1.74 x 10−5 = 10−4.64 x [CH3COO−(aq)] / 1.20
[CH3COO−(aq)] = 1.74 x 10−5 x 1.20 / 10−4.64 = 0.911 mol dm−3
M(CH3COONa) = (2 x 12.01) + (3 x 1.01) + (2 x 16.00) + 22,99 = 82.04 g mol∼1
Mass of CH3COONa required in 50.0 cm3 = 0.911 x 82.04 x 50.0 / 1000 = 3.74 g

 IB Docs (2) Team
IB Docs (2) Team