Relating Topics 4 & 14 to all the other topics
Relating 'Chemical bonding & structure' to the other ten topics
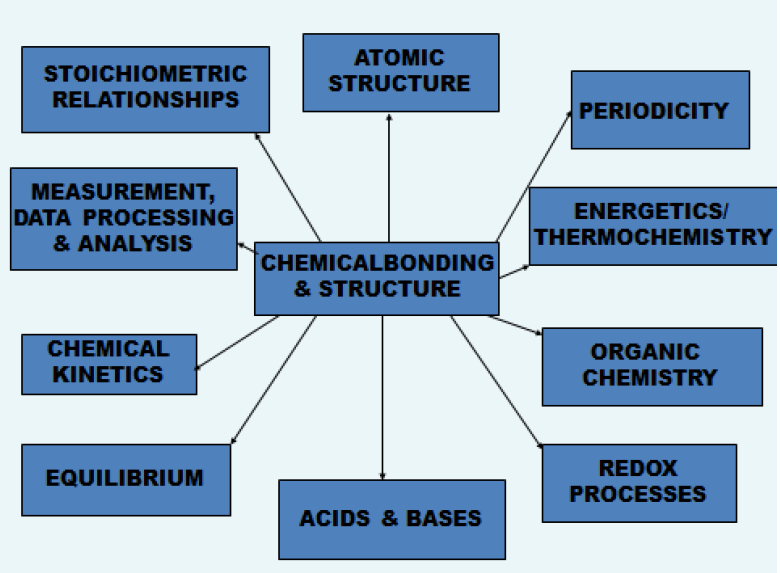
One example at Standard level and one example at Higher Level relating Topics 4 & 14 Chemical bonding & structure to each of the other core and AHL topics is given below. (There are of course many other examples that could have been used to illustrate the relationships between the topics.)
Core relationships
Topic 1: Stoichiometric relationships
![]() &
& ![]() Different types of bonding affect the properties of substances including changes of state (1.1).
Different types of bonding affect the properties of substances including changes of state (1.1).
Topic 2: Atomic structure
![]() Ionic compounds are formed as the result of electron transfer. The electrons that are transferred result in the ions formed having the same electron arrangement as a noble gas (2.2).
Ionic compounds are formed as the result of electron transfer. The electrons that are transferred result in the ions formed having the same electron arrangement as a noble gas (2.2).
Topic 3: Periodicity
![]() In order to predict whether a compound formed between two elements will be covalent or ionic their position in the periodic table and their electronegativities need to be taken into account (3.1 and 3.2).
In order to predict whether a compound formed between two elements will be covalent or ionic their position in the periodic table and their electronegativities need to be taken into account (3.1 and 3.2).
Topic 5: Energetics/thermochemistry
![]() To be able to explain the relationship between the number of bonds and bond strength it is necessary to be able to calculate the enthalpy changes associated with breaking and making bonds (5.3).
To be able to explain the relationship between the number of bonds and bond strength it is necessary to be able to calculate the enthalpy changes associated with breaking and making bonds (5.3).
Topic 6: Chemical kinetics
![]() The rate of a reaction increases as the temperature is increased because the extra energy increases the likelihood of the shared pair of electrons in a covalent bond having the necessary activation energy to split apart when molecules collide (6.1).
The rate of a reaction increases as the temperature is increased because the extra energy increases the likelihood of the shared pair of electrons in a covalent bond having the necessary activation energy to split apart when molecules collide (6.1).
Topic 7: Equilibrium
![]() Resonance structures such as those shown in benzene and the carbonate anion are not in dynamic equilibrium (7.1) with each other. Resonance structures are extreme forms and the true structure lies between the resonance structures.
Resonance structures such as those shown in benzene and the carbonate anion are not in dynamic equilibrium (7.1) with each other. Resonance structures are extreme forms and the true structure lies between the resonance structures.
Topic 8: Acids & bases
![]() When weak acids dissociate in aqueous solution (8.4) a covalent bond needs to be broken to form a hydronium ion, H3O+ and the acid radical.
When weak acids dissociate in aqueous solution (8.4) a covalent bond needs to be broken to form a hydronium ion, H3O+ and the acid radical.
Topic 9 Redox processes.
![]() The formation of ionic compounds (such as NaCl or MgO) from their elements involves the transfer of electrons so that it can always be classified as a redox reaction. The species that has lost electrons is oxidized and the species that has gained electrons is reduced (9.1).
The formation of ionic compounds (such as NaCl or MgO) from their elements involves the transfer of electrons so that it can always be classified as a redox reaction. The species that has lost electrons is oxidized and the species that has gained electrons is reduced (9.1).
Topic 10: Organic chemistry
![]() Nucleophiles are electron-rich species that contain a non-bonding pair of electrons that they donate to an electron-deficient carbon (10.2). The donated pair of electrons form a coordinate bond.
Nucleophiles are electron-rich species that contain a non-bonding pair of electrons that they donate to an electron-deficient carbon (10.2). The donated pair of electrons form a coordinate bond.
Topic 11: Measurement & data processing
![]() The relative polarity of bonds can be predicted from electronegativity values. These values are not exact and there are different scales of measurement. Pauling’s scale gives the values to one decimal place whereas some other other values used in periodicity (e.g. atomic mass) are given to two decimal places. (11.1).
The relative polarity of bonds can be predicted from electronegativity values. These values are not exact and there are different scales of measurement. Pauling’s scale gives the values to one decimal place whereas some other other values used in periodicity (e.g. atomic mass) are given to two decimal places. (11.1).
AHL relationships
Topic 12: Atomic structure
![]() The wavelength of light required to dissociate oxygen and ozone (14.1) can be calculated using the equation E = hν (12.1).
The wavelength of light required to dissociate oxygen and ozone (14.1) can be calculated using the equation E = hν (12.1).
Topic 13: The periodic table - the transition metals
![]() Explanation of the nature of the coordinate bond within a complex ion (13.1).
Explanation of the nature of the coordinate bond within a complex ion (13.1).
Topic 15: Energetics/thermochemistry
![]() Construction of Born-Haber energy cycles and their use (15.1) to explain the processes occurring during the formation of an ionic bond.
Construction of Born-Haber energy cycles and their use (15.1) to explain the processes occurring during the formation of an ionic bond.
Topic 16: Chemical kinetics
![]() Catalysts alter a reaction mechanism, introducing a step with lower activation energy (16.1) by forming a chemical or physical bond with one or more of the reactants.
Catalysts alter a reaction mechanism, introducing a step with lower activation energy (16.1) by forming a chemical or physical bond with one or more of the reactants.
Topic 17: Equilibrium
![]() The position of equilibrium for any reaction involving the breaking and formation of chemical bonds corresponds to a maximum value of entropy and a minimum in the value of the Gibbs free energy (17.1).
The position of equilibrium for any reaction involving the breaking and formation of chemical bonds corresponds to a maximum value of entropy and a minimum in the value of the Gibbs free energy (17.1).
Topic 18: Acids & bases
![]() When a Lewis base reacts with a Lewis acid a coordinate bond is formed (18.1).
When a Lewis base reacts with a Lewis acid a coordinate bond is formed (18.1).
Topic 19: Redox processes
![]() Current, duration of electrolysis and charge on the ion affect the amount of product formed at the electrodes during the electrolysis of a molten or aqueous ionic substances (19.1).
Current, duration of electrolysis and charge on the ion affect the amount of product formed at the electrodes during the electrolysis of a molten or aqueous ionic substances (19.1).
Topic 20: Organic chemistry
![]() A chiral carbon atom is a carbon atom covalently bonded to four different atoms or groups (20.3).
A chiral carbon atom is a carbon atom covalently bonded to four different atoms or groups (20.3).
Topic 21: Measurement & analysis
![]() The structural technique of single crystal X-ray crystallography can be used to identify the bond lengths and bond angles of crystalline compounds 21.1).
The structural technique of single crystal X-ray crystallography can be used to identify the bond lengths and bond angles of crystalline compounds 21.1).

 IB Docs (2) Team
IB Docs (2) Team