Hybridization
 14.2 Hybridization (2 hours)
14.2 Hybridization (2 hours)
Pause for thought
The concept of hybridization is a useful model to explain the shape, bond angles and Lewis structure of molecules. It was developed by Linus Pauling in 1932, the same year that he introduced the idea of electronegativity. I first came across it at university and it has a somewhat chequered history in pre-university syllabuses. During the 1970s and 1980s it appeared on British A level syllabuses but it has subsequently disappeared from most pre-university syllabuses with the IB being somewhat of an exception.
Essentially hybridization describes the combination of atomic orbitals to form new hybrid atomic orbitals with the same mean energy. In the IB this is limited to sp, sp2 and sp3 but at a university level it is also involves the hybridization of d orbitals. It can be used to describe shapes in coordination complexes of transition metal compounds such as [Mn(H2O)6]2+ where the hexagonal-shaped complex ion can be explained by invoking d2sp3 hybridization on the metal ion.
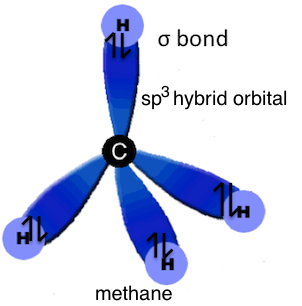
sp3 hybridization used to explain the symmetrical tetrahedral shape of methane where a single electron in each of the sp3 hybrid orbitals on the carbon atom forms a sigma bond with the single s electron in each of the four hydrogen atoms.
Nature of science
Theories are often uncertain. For example, hybridization is part of valence bond theory and can help to explain molecular geometries and some chemical behaviour but is limited. Another example is quantum mechanics which involves several theories. These can explain the same phenomena but depend upon specific requirements.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesOnly species with sp3, sp2 and sp hybridization need to be considered. (Note that this should include benzene and non-carbon containing compounds such as ammonia as well as alkanes, alkenes and alkynes.) International-mindedness(Nothing is listed in the programme for international-mindedness under this sub-topic) |
Teaching tipsHybridization is one of the areas of the syllabus that students find most difficult. Having first explained about the shapes of the p and s orbitals and the linear combination of atomic orbitals to form molecular orbitals. I then ask students to tell me all they know about methane and elicit from them the fact that it contains four equal C—H bonds in a tetrahedral shape. How can this be possible when the electron configuration of carbon is 1s22s22p2? There are only two unpaired electrons so perhaps carbon should have a valency of two? This is not the case, although it is true further down group 4, particularly for lead. If one of the s electrons is promoted to a p orbital we now have four unpaired electrons so we can get four bonds with hydrogen but they will not all be equal and the resulting shape would not be tetrahedral. Promoting an electron from an s to a p orbital requires energy but this can be accounted for by hybridizing the orbitals to give four new sp3 hybrid orbitals with a lower average energy than separate s and p orbitals so that there is no mean energy change. It is then relatively easy to proceed to use ethene and benzene to illustrate sp2 hybridization and ethyne to illustrate sp hybridization. Once students can see that sp3 results in a bond angle of 109.5o, sp2 gives 120o and sp 180o they can then work backwards for other compounds involving other elements. For example nitrogen in the ammonium ion must be sp3 hybridized as the shape is tetrahedral and the bond angle is 109.5o. It is worth stressing that hybridization is a useful theory - but it is only a theory. Because students seem to find this topic difficult I have writen more about it under Understanding hybridization in Areas of difficulty for exams where I have also listed and given examples of the type of questions that can be asked on hybridization. | Study guide
Pages 34 & 35 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Hybridization. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Hybridization questions. Vocabulary listhybridization IM, TOK, 'Utilization' etc.See separate page which covers all of Topics 4 & 14. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. One of the better videos on hybridization (there are many bad ones on YouTube!). This explains sp (BeCl2), sp2 (benzene) and sp3 (methane) hybridization correctly in terms of energy as well as shapes.
2. A video that shows rotating models of the different types of hybridization between s and p orbitals.

 IB Docs (2) Team
IB Docs (2) Team 













