Voltaic cells
Introduction and teacher's notes
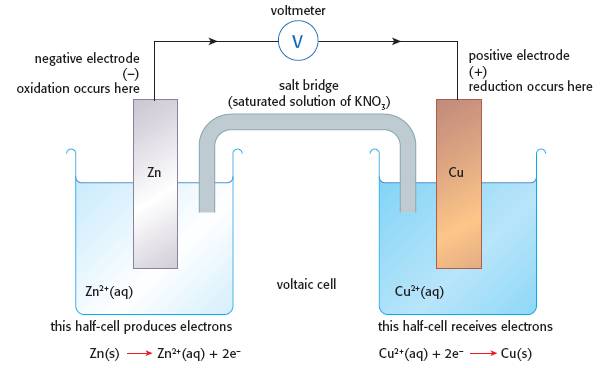
There is actually very little on the core part of the syllabus about voltaic cells under Topic 9.2 but it does contain the mandatory laboratory component in the Applications and skills section, "Performance of laboratory experiments involving a typical voltaic cell using two metal/metal-ion half-cells". All that Standard Level students really need to know is how two half-cells (e.g. Mg(s), Zn(s), Fe(s) and Cu(s) in solutions of their own ions) can be connected by a salt bridge to produce electricity by undergoing a spontaneous reaction and draw a diagram to show it (see above). They also need to be able to state that oxidation occurs at the negative electrode (anode) and reduction occurs at the positive electrode (cathode). This practical demonstrates all this and they can also gain an understanding of the concept of an activity series through it. It does go further as it includes the concept of equilibrium and what happens if the concentrations of the salt solutions changes. Students can be encouraged to think out what will happen by using Le Chatelier’s Principle which also helps to address some of the content of Topic 7.1 on Equilibrium. For Higher Level students the use of Standard Electrode Potentials, E⦵, can also be included and it can enable them to predict which reaction will occur when two half-cells are connected together. You could also get them to calculate the free energy of the cell when it is operating using the expression ΔG⦵ = − nFE⦵ (see Topic 19.1) but the use of the Nernst equation is only required in Option C: Energy.
It is a very safe practical to carry out although the solutions should be disposed of in the ‘Heavy Metal’ waste container afterwards. It does not take long but it is well worth doing as they gain a good understanding by making them think about each step. They can see that cell convention is not necessary as it does not matter which cell is placed on the left or which on the right. The voltage produced by the cell can be seen to be dependent only on the relative reactivities of the metals with the metal higher in the reactivity series (or electrochemical series for HL) forming the negative electrode. This practical could be used as Scaffolding to prepare students who want to bring some aspect of voltaic cells into their Individual Scientific Investigation.

 Student worksheet
Student worksheet
VOLTAIC CELLS
This experiment demonstrates how a chemical reaction can be arranged to give out energy in the form of electricity and looks at some of the factors determining the value of E⦵cell.
ENVIRONMENTAL CARE:
Do not pour solutions of heavy metal ions (Cu2+, Fe2+ and Zn2+) down the sink. Place all residues in the waste bottles provided in the fume cupboard.
SAFETY:
This experiment does not involve any particular safety hazards.
PROCEDURE:
 1. Construct a zinc / copper cell using 1.0 mol dm−3 solutions of Cu2+ and Zn2+ ions and strips of coppers and zinc with the two half cells connected by a salt bridge as shown in the photograph on the right. Measure the cell e.m.f. using a high resistance voltmeter and try to determine which way the electrons are flowing in the external circuit.
1. Construct a zinc / copper cell using 1.0 mol dm−3 solutions of Cu2+ and Zn2+ ions and strips of coppers and zinc with the two half cells connected by a salt bridge as shown in the photograph on the right. Measure the cell e.m.f. using a high resistance voltmeter and try to determine which way the electrons are flowing in the external circuit.
2. From the relative tendencies of zinc, iron and copper to lose electrons predict whether you would expect a zinc / iron cell to have a greater or smaller Ecell than the zinc / copper cell. Test your prediction by constructing a zinc / iron cell using the same zinc half-cell connected to an iron half-cell made with a piece of iron and a 1.0 mol dm−3 solution of Fe2+. Assuming that each half-cell makes a fixed independent contribution to E⦵cell predict what the value of E⦵cell will be for an iron / copper cell under the same conditions. Test your prediction by constructing an iron / copper cell made from the two half-cells used previously. How does your measured E⦵cell value compare with your predicted value?
3. The total e.m.f. produced by a cell depends on several factors, one of them is concentration. When current is drawn from a zinc / copper cell the reactions occurring at each electrode are:
Zn(s) → Zn2+(aq) + 2e− and Cu2+(aq) + 2e− → Cu(s)
However when the e.m.f. is being measured no current is being drawn and each electrode is at equilibrium.
Zn(s) ⇌ Zn2+(aq) + 2e− and Cu2+(aq) + 2e− ⇌ Cu(s)
Use Le Chatelier's Principle (the Equilibrium Law) to predict the changes which occur if the ion concentration in each equilibrium is reduced. What will be the effect of decreasing the concentration of copper ions on the tendency of the copper electrode to accept electrons from the zinc? What effect will this have on E⦵cell? Test your prediction by setting up a zinc / copper cell using the zinc half-cell previously used above but with the following concentrations of Cu2+(aq), 0.100, and 0.001 mol dm−3, making sure to rinse the salt bridge with distilled water each time.
QUESTIONS
1. What are the standard conditions under which E⦵cell values are usually measured?
2. Using the E⦵ values from the data book compare the values you obtained with the standard values.
3. What value might you expect for a cell made from your standard copper half-cell connected to a zinc half-cell where the concentration of zinc ions was 0.001 mol dm−3?
4. When the reaction between Zn(s) and Cu2+(aq) reaches equilibrium what will Ecell equal?
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team