Carbohydrates

 B.4 Carbohydrates (2 hours)
B.4 Carbohydrates (2 hours)
Pause for thought
Stereochemistry of glucose
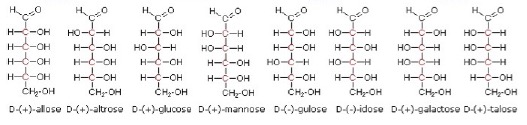
Although glucose is a simple sugar and a well-known compound, its structure and how it can be represented does pose some problems. It contains four chiral centres. This means that theoretically 24 (i.e. 16) stereoisomers are possible, arranged in eight diastereoisomeric pairs. This was first recognised by Emil Fischer, a German chemist, who won the Nobel Prize for chemistry in 1902. Fischer arbitrarily labelled any glucose structure where the –OH bonded to the last chiral carbon atom next to the aldose group (furthest from the aldehyde group) on the right as the D- form. This is for convenience and does not relate to whether the stereoisomer is the d- or l- form in terms of the rotation of plane polarized light. The eight possible stereoisomers of the D form, drawn in what are known as Fischer structures are thus:
In solution the –OH group on the fifth carbon atom forms an ether linkage with the first carbon atom and the carbonyl group is converted into an alcohol. If the –OH group formed on the first carbon atom is on the opposite side of the ring to the sixth carbon atom then it is known as the alpha- form.
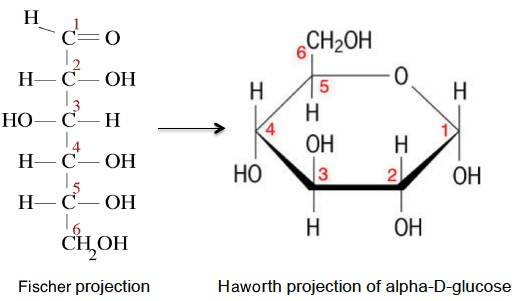
The syllabus uses the Haworth projection, which is a convenient way to show the stereochemistry. The ring drawn in the chair conformation shows both the conformation and stereochemistry but is not required by the IB.

Nature of Science
Understanding the stereochemistry of carbohydrates is the key to understanding their structural roles in cells. By making carbon and hydrogen implicit, models using Haworth projections help to focus on the nature and position of attached groups.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe straight chain and α-ring forms of glucose and fructose can be found Section 34 of the data booklet. The following are not required: International-mindednessSugar is a major international commodity that is produced in about 130 different countries. About three-quarters of the production comes from sugar cane in tropical and subtropical regions with the remainder coming from sugar beet that is cultivated in temperate climates.The World Health Organization projects that deaths from diabetes (a chronic disease that occurs when the body cannot effectively regulate blood sugar, due to a failure in the production or functioning of insulin) will double between 2005 and 2030. Lactose intolerance is due to a failure to produce sufficient levels of lactase, the enzyme that hydrolyses lactose into glucose and galactose. Globally, lactose intolerance (a condition in which the individual is not able to digest lactose, the sugar found in milk and dairy products) is the norm and provides an example of a Western perspective invading science. |
Teaching tipsThis can be quite a difficult topic to teach, particularly the isomers of glucose but hopefully you can put the information in ‘Pause for though above’ to good use. Start by looking at the different forms of monosaccharides then go on to stress the loss of water during their condensation reactions to form different types of di- and polysaccharides In fact, the condensation reactions of sugars have already been covered in B.1: Introduction to biochemistry so can be reinforced here. If you can get students to make three-dimensional models using a modelling kit such as Molymod it will aid their understanding of the structures of sugars, how they cyclise (and also the difference between the alpha- and beta- forms although this distinction is not actually required) and which linkages are formed when they undergo condensation reactions. The functions of carbohydrates (including, energy source, storage of energy in the form of glycogen, dietary fibre and as biological precursors) are mainly just memorising although their many polar hydroxyl groups an be used to explain their solubility in water. Stress that carbohydrates are not such a good energy source (weight for weight) as fats as they are already in a partially oxidized form. An example of their use as a precursor is their role in the biosynthesis of proteins as they are components of nucleic acids. | Study guidePage 130 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Carbohydrates. For short-answer questions see Carbohydrate questions together with the worked answers on a separate page Carbohydrate answers. Vocabulary list:monosaccharide Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A simple video (possibly made by students) but it provides a good illustration of the structures of glucose and condensation reactions to form starch, glycogen and cellulose.
2. A lecture on the ring structures of sugars including the Haworth projection.
3. A link to a website from Elmhurst College that helps to distinguish clearly between the amylose and amylopectin forms of starch. It also contains links to other web pages on carbohydrates.

 IB Docs (2) Team
IB Docs (2) Team 













