Determination of vitamin C content

 Introduction
Introduction
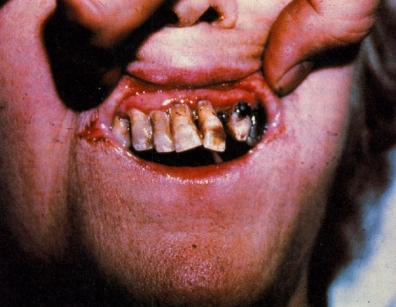 Vitamin C is one of the vitamins listed in B.5 Vitamins. You will need to discuss its structure and explain why it is water-soluble as well as discuss the causes and effects of a lack of vitamin C in the diet. The picture taken from Man-of-War by Stephen Biesty (Dorling-Kindersley, NY, 1993) graphically shows the effects of scurvy on the gums and teeth! The recommended daily allowance is about 60-70 mg and this can be obtained easily by eating fresh fruit and vegetables. However many people do also take supplementary vitamin pills (perhaps because they erroneously think that it will help to ward off the common cold - see the page which includes the section on Linus Pauling and the ad hominen fallacy).
Vitamin C is one of the vitamins listed in B.5 Vitamins. You will need to discuss its structure and explain why it is water-soluble as well as discuss the causes and effects of a lack of vitamin C in the diet. The picture taken from Man-of-War by Stephen Biesty (Dorling-Kindersley, NY, 1993) graphically shows the effects of scurvy on the gums and teeth! The recommended daily allowance is about 60-70 mg and this can be obtained easily by eating fresh fruit and vegetables. However many people do also take supplementary vitamin pills (perhaps because they erroneously think that it will help to ward off the common cold - see the page which includes the section on Linus Pauling and the ad hominen fallacy).
Teacher's notes
Because vitamin C is easily oxidised (it is a good antioxidant) its percentage composition in a sample can be determined easily by titrating it against an oxidising agent such as DCPIP (2,6-dichlorophenol-indophenol) or iodine. This experiment uses iodine. In order to make it a little more challenging the concentration of the iodine solution given to the student is unknown so the first step for them is to standardise the iodine solution. This practical is suitable for both SL and HL students. SL students will need more guidance to obtain the correct redox equations so these should probably be given. The calculation is not straightforward as it involves several redox reactions and could be good practice when it comes to students solving problems using the Winkler method to measure biochemical oxygen demand (covered in 9.1 Oxidation & reduction) as that too involves following through several redox reactions.
Most teachers will probably use this practical if they are teaching Option B, or if their students want to study some aspect of vitamin C for their Extended Essay or Individual Scientific Investigation. It could actually be used to cover the Mandatory laboratory component Topic 1.3. Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard solution.
You (or your technician) will need to prepare three separate solutions.
Iodine solution. This should be approximately 0.050 mol dm-3 and can be made by 12.8 g of iodine and 40 g of potassium iodide in water and making up to one litre.
0.100 mol dm-3 sodium thiosulfate solution.
1% starch solution. Mix 1 g of starch into a paste with a little water then add 100 cm3 of boiling water.
Use a 1 g vitamin C tablet.

 Student worksheet
Student worksheet
DETERMINATION OF VITAMIN C CONTENT
INTRODUCTION
Vitamin C is present in many fruits and vegetables. It is necessary part of the diet as it is required to form the protein, collagen. A lack of vitamin C can cause lesions in the skin – a condition known as scurvy. To ensure a sufficient intake of vitamin C some people take vitamin supplements.
Vitamin C has the formula C6H8O6 and can be oxidised to form C6H6O6. The amount of vitamin C present in a vitamin tablet can therefore be determined by titrating a known amount of the tablet with an oxidising agent. In this experiment iodine, I2(aq) is used as the oxidising agent. The iodine provided is of unknown concentration so that the first step of the experiment is to standardise the iodine solution using standard sodium thiosulfate solution, Na2S2O3(aq).
ENVIRONMENTAL CARE:
The small amounts used in this experiment do not contain any seriously harmful substances to the environment once diluted and can be disposed of safely down the sink.
SAFETY:
Avoid skin contact with the iodine solution and be sure to wear safety glasses to avoid getting any iodine in your eyes.
PROCEDURE:
(a) To standardise the iodine solution.
Pipette 10.0 cm3 of the 0.100 mol dm-3 sodium thiosulfate solution provided into a conical flask and add a few drops of freshly prepared starch solution. Titrate with the iodine solution provided until one drop causes the blue-black colour to remain permanently. Repeat the procedure to obtain two accurate results.
(b) To determine the percentage of vitamin C.
Record the mass of a vitamin C tablet and then dissolve it in approximately 50 cm3 of distilled water. Transfer the solution and all the washings into a 100 cm3 volumetric flask and make up to the mark with distilled water. Pipette a 10.0 cm3 aliquot of this solution into a conical flask and add a few drops of freshly prepared starch solution. Titrate with the solution of iodine until one drop causes the blue-black colour to remain permanently. Repeat the procedure to obtain two accurate results
Record your results in a suitable format. From your results follow the steps below to determine the concentration of the iodine solution used and hence the percentage of vitamin C present in the tablet including all the uncertainties.
CALCULATION
The equation for the reaction of sodium thiosulfate with iodine is:
I2(aq) + 2Na2S2O3(aq) → 2NaI(aq) + Na2S4O6
1. Determine the amount (in mol) of sodium thiosulfate in 10.0 cm3 of 0.100 mol dm−3 Na2S2O3(aq).
2. Determine the amount of iodine (in mol) in the average volume of I2(aq) used in the titration with Na2S2O3(aq).
3. Determine the concentration (in mol dm-3) of the iodine solution.
The half-equation for the reduction of iodine is:
I2(aq) + 2e– → 2I–(aq)
and the half-equation for the oxidation of vitamin C is:
C6H8O6(aq) → C6H6O6(aq) + 2H+(aq) + 2e–
4. Give the overall equation for the reaction of vitamin C with iodine.
5. Calculate the amount of iodine (in mol) in the average volume used to react with the vitamin C solution.
6. Calculate the amount of vitamin C in the 10.0 cm3 aliquot of vitamin C solution
7. Calculate the amount of vitamin C present in the vitamin C tablet.
8. Calculate the percentage by mass of vitamin C in the vitamin C tablet.
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team