Key terms & concepts
Rationale
Each sub-topic in the detailed syllabus content given in the IB Guide starts with a statement on how the sub-topic can relate to the Nature of Science. If these are considered in total then certain themes and concepts tend to repeat themselves. Hopefully as you learn your teacher will bring in references as to how particular topics illustrate or relate to the Nature of Science. It might also be worth looking at the pages given by Berkeley University on Understanding Science. There is also a good TED talk by Stuart Firestein on how Science works entitled 'The Pursuit of Ignorance' which talks about the real scientific method rather than the accepted one.
When it comes to answering questions in the examination the NOS questions tend to be set in a chemistry context rather than standalone questions. I think if you know the key terms and concepts used in the Nature of Science then you should have little difficulty in answering the questions and perhaps more importantly have a much better understanding of how science works. The list is not exhaustive but should provide a good basis.
Key terms & concepts
Assumptions. Sometimes chemistry is based upon assumptions even though they are not true in order to provide a useful model. For example to determine the oxidation states of elements in a molecule like ammonia you assume it is ionic with the ‘anion’ being the element, in this case nitrogen, with the higher electronegativity so the oxidation state of nitrogen is – 3 and the oxidation state of hydrogen + 1. For the simple octet rule and to determine shapes using VSEPR theory it is assumed that all valence electrons are the same, the fact that there are s and p electrons in different energy levels is ignored. Oxidation is commonly defined as the loss of electrons and yet when carbon burns in oxygen neither carbon nor oxygen loses electrons.
Changing theories. Theories change to accommodate new information and understanding as chemistry develops. The different theories of acids provide a good example of this. In Roman times acids were defined as sour substances, then the oxide of a non-metal in water by Lavoisier in the 1780s, then a substance that donates protons by Lowry-Brønsted in 1923 before being defined as a substance that accepts a pair of electrons by Lewis also in 1923. An even later definition by Usanovich in the 1930s defines an acid as a substance that accepts negative species or donates positive species.
Collaboration and collusion. There are many examples of scientists collaborating. This often occurs across international boundaries and also provides a good example of international-mindedness. Classic examples of this include CERN at Geneva where recently evidence for the Higgs-Boson particle has been obtained and on a much smaller scale the IB Diploma Chemistry programme is a result of several chemists (teachers and researchers) from around the world collaborating to provide a truly international programme. Collaboration is for the common good; collusion is the opposite and refers to a secret agreement between two or more parties for fraudulent, illegal or deceitful purposes. One of the most recent examples of collusion involved the manipulation of data to confirm global warming at the University of East Anglia’s Climate Research Unit.
Ethical implications. The story of Fritz Haber who won the Nobel Prize for discovering how to fix nitrogen and thus provide artificial fertilisers to feed the world and yet who also worked on chlorine as a poison gas in World War 1 is a classic illustration of the ethical problems facing chemists. Energy is needed by society but almost all the ways in which it is generated are also bad for society. Green chemistry is one way in which chemists try to act responsibly towards society and yet still make scientific advances and supply goods such as drugs and pesticides which are helpful to society.
Falsification. Karl Popper in the 1950s maintained that it is impossible to prove something by doing an experiment as you would need to do an infinite number of experiments to cover every possible permutation. However if you try to disprove a theory you only need one successful experiment to disprove it. He maintained that a theory is only scientific if it is capable of being falsified. pV = nRT is only true for an ideal gas; real gases do occupy some volume and do have some weak intermolecular forces of attraction. Lavoisier’s theory of acids was falsified when scientist realized that HCN and HCl do not contain oxygen.
Instrumentation. Modern instrumentation has revolutionized the way chemists work. For example, it is now possible to determine very small quantities of enhancing drugs in athletes who cheat, to catch criminals from DNA residues and to manipulate the addition of precise amounts of metals during the production of alloys to achieve the desired properties. It has also enabled chemists to unambiguously assign structures quickly to new compounds.
Language of chemistry. Chemists communicate to each other in very precise language. Sometimes the meaning of a word is different to its use in everyday English. For example, spontaneous in chemistry means that the reaction is able to do useful work, i.e. ΔG has a negative value. In everyday English spontaneous means without preplanning, i.e. ‘off the cuff’. Some examples of other words with different meanings to chemists are strong, reduce, degenerate, weak, mole, phase and volatile. Chemists need to be aware that, unlike other chemists, the general public may misinterpret these words if they are used in the chemical sense. A strong drink or a weak acid means something different to a chemist than it does to everyone else. There are other ways in which the language of chemistry is precise. The IUPAC naming of organic compounds follows clearly defined rules and the oxidation numbers of elements is shown using Roman numerals in inorganic compounds, e.g. iron(III) chloride. Other language rules include writing physical constants in italics, e.g. equilibrium constants should be written as Kc or pKa etc., and using lower case letter to write the names of elements and compounds in sentences even though their chemical symbols start with a capital letter. For more on this see Language of Chemistry in the TOK section.
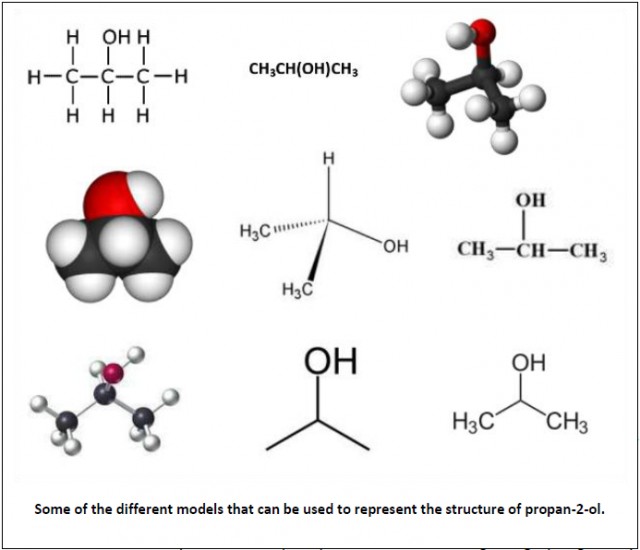
Models. Chemists use a variety of molecular models to represent the structure of molecules (e.g. ball and stick, space filling etc.) and more sophisticated computer modelling to represent systems where there are many different variables such as climate change. Many potential drugs are first made virtually and modelled to see whether they might be effective before synthesizing those which look as if they may have some potential to test in vitro and then in vivo.
Occam’s razor. This basically states that simple explanations are, other things being equal, generally better than more complex ones. For example collision theory is very simple but based on models of reacting species it explains kinetic theory and the factors affecting the rate of chemical reactions. One example is given wrongly in the syllabus under sub-topic 8.3 pH scale is not an example of Occam's razor as it does not attempt to explain anything. It is simply using a logarithmic scale to express hydrogen ion concentrations in solution.
Paradigm shift. Thomas Kuhn proposed that scientific progress works through paradigms. This is an established model accepted by the scientific community. As more becomes known the paradigm has to accommodate to fit the new knowledge. Eventually it becomes unwieldy and a new model becomes accepted - a paradigm shift. The classic old example in chemistry is phlogiston, a substance which was thought to be given off by everything when it burned. Even when Priestly discovered oxygen he called it ‘dephlogisticated air’ as substances readily gave up phlogiston to it. It took the genius of Lavoisier to explain that combustion occurs when substances combine with oxygen rather than give off a substance. A more modern paradigm in chemistry is simple covalent bonding using the octet rule. Sharing a pair of electrons (one from each atom) to form a share in an inert gas structure works for simple substances like hydrogen, methane, carbon dioxide etc. It has to be stretched to include coordinate bonding and resonance hybrids but cannot explain substances like SF6 or NO. A new paradigm is molecular orbital theory or valence bond theory which takes account of the fact that electrons are in different orbitals and energy levels and also explains why diatomic oxygen is paramagnetic. Another example of a paradigm shift is the change from the understanding that atoms are indivisible to the paradigm in which they can be broken into many different sub-atomic particles.
Patterns, trends and discrepancies. There are many observable patterns in chemistry and scientists make predictions based upon them. The most obvious example is the periodic table. By leaving spaces to accommodate the known elements Mendeleev was able to predict the properties of elements which had yet to be discovered with remarkable accuracy. Often trends are apparent but there are discrepancies. New theory and explanations often emerge to explain these discrepancies. For example the transition elements follow a certain pattern of behaviour but chromium and copper do not follow this pattern and this can be explained by the fact that the 4s orbital in the free atom is only occupied by one electron not two. Another example is that primary amines are more basic than ammonia and secondary amines are more basic than primary amines. This trend is ascribed to the inductive effect of the R- groups attached to the central nitrogen atom. However tertiary amines (which have three R- groups attached to the nitrogen atom) do not follow this pattern and are less basic than secondary amines. This may be due to the fact that the nitrogen atom no longer has a hydrogen atom bonded to it so tertiary amines are not involved in hydrogen bonding.
Predictions. A good scientific theory enables scientist to make predictions. For example, theory explains that the lines in the visible emission spectrum of hydrogen are due to excited electrons dropping from higher energy levels to the n=2 level. If this is true one can predict that there should be another series of lines at higher energy corresponding to electrons dropping to the lower n=1 level. This cannot be seen by the naked eye but the series is there if you use an ultraviolet spectrometer. Similarly there are more series at lower energy in the infrared region due to electrons dropping to the n=3 and n=4 levels etc.
Serendipity. The accidental discovery of something useful when not looking for it. Legend has it that glass was discovered when Phoenician sailors cooked a meal on a sandy beach. Liquid crystals were discovered by Friedrich Reinitzer when he was doing experiments with cholesteryl benzoate, William Perkin discovered azo-dyes when he was trying to synthesise quinine and Alexander Fleming is credited with discovering penicillin when he was working with staphylococci bacteria. Other examples include the discovery of Teflon, poly(tetrafluoroethene), and superglue.
Use of concepts. Chemists often use concepts to work out values that cannot be determined directly. Energy cycles are a good example of this because they are based on the First Law of Thermodynamics - energy can neither be created no destroyed. It is easy to determine the enthalpies of combustion of carbon, hydrogen and methane practically. It is impossible to determine the enthalpy of formation of methane directly as carbon and hydrogen can react together to form many different compounds. However by using an energy cycle the value for the enthalpy of formation can be readily obtained indirectly.

 IB Docs (2) Team
IB Docs (2) Team