8.4 Strong & weak acids & bases
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 8.4 Strong & weak acids & bases. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.


 Learning outcomes
Learning outcomes
 After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- Strong acids and bases differ to weak acids and bases in the extent to which they are ionized in aqueous solution.
- The conductivity of solutions of strong acids and bases is higher than the conductivity of solutions of weak acids and bases with the same concentration.
- Strong acids are good proton doors and have weak conjugate bases.
- Strong bases are strong proton acceptors and have weak conjugate acids.
Apply your knowledge to:
- Distinguish between strong and weak acids and bases in terms of the different rates of their reactions with metals, metal oxides, metal hydroxides, metal hydrogen carbonates and metal carbonates and in terms of their electrical conductivities when the concentrations of their solutions are equal.
Relationships & vocabulary
Nature of science
As a result of improved instrumentation advanced analytical techniques have meant that the relative strengths of different acids and bases can be quantified. This has enabled trends and discrepancies (patterns and anomalies) to be found and explained at the molecular level. Further evidence that weak acids exist in equilibria can be obtained from experiments and models
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 8 & 18: Acids & bases.
Vocabulary
| strong acid / strong base | weak acid / weak base | electrical conductivity | dissociation constant |
| ionization constant | Ka and pKa | Kb and pKb |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
This sub-topic is really a very basic introduction to the differences between strong and weak as applied to acids and bases. Where it can get slightly tricky is if you are asked to determine the relative strengths of acids and bases from experimental data. This data might be conductivity measurements or more likely the extent of dissociation in water. Standard Level students should understand about acid (and base) dissociation constants as equilibrium constants are covered in the previous topic on equilibrium. but they are not expected to know specifically about pKa and pKb. It would not be unreasonable to give Standard Level students the values of Ka for two different weak acids and ask them which is the strongest.
The problem is that Section 21 in the IB data booklet is specifically referred to in the 'Guidance' section of this sub-topic 8.4 (see below). This Section lists the values for the strengths of common acids and bases, but it only gives them as pKa or pKb values even though pKa and pKb are not on the Standard Level programme.
Some examples from the IB data booklet are given below:
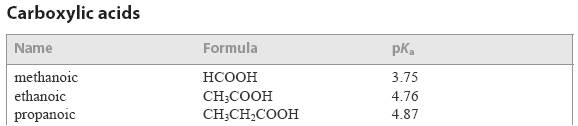

Without necessarily understanding why, if you are a standard level student you need to know that the greater the pKa or pKb value the weaker (i.e. less dissociated) the acid or base. So that methanoic acid is a stronger acid than ethanoic acid and ammonia is a weaker base than methylamine.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Strong & weak acids & bases.
For short-answer questions see Strong & weak acid & bases questions.
More resources
1. Richard Thornley's laser gun explains the concept of strong and weak when applied to acids and bases.
Strong & weak acids & bases (1) ![]()
2. Jonathan Bergmann and Aaron Sams interactively talk about strong & weak acids & bases.
Strong & weak acids & bases (2) ![]()

 IB Docs (2) Team
IB Docs (2) Team 














