D.6 Environmental impact of some medications
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option D - sub topic D.6. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.


 Learning outcomes
Learning outcomes
 After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- High-level waste (HLW) is radioactive waste that emits large amounts of ionizing radiation for a long period of time.
- Low-level waste (LLW) is radioactive waste that emits small amounts of ionizing radiation for a short period of time.
- Resistance to antibiotics occurs when micro-organisms become resistant to antibacterials.
- Describe the environmental impact of the disposal of radioactive medical waste.
- Discuss the environmental issues related to left-over solvents.
- Explain the dangers associated with antibiotic waste, from the improper disposal of drugs and animal waste, and the development of antibiotic resistance.
- Discuss the basics of green chemistry (sustainable chemistry) processes.
- Explain how green chemistry was used to develop the precursor for oseltamivir (Tamiflu).
Relationships & vocabulary
Nature of science
The design, production and distribution of medications involve considerable ethical implications and risks which need to be considered by the whole community as well as by the scientific community. Both the side effects of medications on the patient and the side effects of the development, production and use of medications on the environment (i.e. disposal of nuclear waste, solvents and antibiotic waste) need to be taken into account.International-mindedness
Pharmaceutical companies are multi-national companies. How do they determine how to spend their funds on research to develop new medications? For example, do they have a responsibility to research medications for rare diseases, even though it is unlikely to provide them with significant financial profit?The production of a drug typically involves a number of different organic reactions. Consider the ethics governing the synthesis of new drugs. Are the standards and practices relating to the design and mIB Docs (2) Teamfacture of pharmaceutical products the same worldwide, or do they vary by country and region?
Vocabulary
| low-level nuclear waste, LLW | high-level nuclear waste, HLW | green chemistry | atom economy | precursor |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Supercritical carbon dioxide
Solvents are traditionally volatile organic substances which are toxic to all living organisms. They affect the nervous and respiratory systems and many, particularly chlorinated solvents such as dichloromethane, affect the liver and kidneys. Many also contribute to photochemical smog by forming secondary pollutants and also some are greenhouse gases and/or contribute towards ozone depletion. Some may also pollute ground water. The pharmaceutical industry requires large quantities of solvents to produce drugs on an industrial scale and much of the solvents used ends up as waste.
Companies are more and more switching to using supercritical carbon dioxide in place of traditional organic solvents. Supercritical carbon dioxide is now used in the extraction of caffeine and essential oils from plant materials, and to extract the required product(s) during the multi-stage synthesis of drugs. This has many advantages. Supercritical carbon dioxide is much greener and safer to the environment than traditional solvents such as propanone or dichloromethane. The extractions can also occur at much lower temperatures than, for example, using steam distillation, which means that the desired products are less likely to be thermally decomposed or denatured during the extractions. Supercritical carbon dioxide is also very easy to remove. It is true that, when released into the atmosphere, carbon dioxide is a greenhouse gas, but it is not adding to the total amount of greenhouse gases if the source of the supercritical carbon dioxide was the atmosphere in the first place.
So what is supercritical carbon dioxide? Chemistry teachers are familiar with carbon dioxide gas and solid carbon dioxide, “dry ice”, but most will not have come across ‘liquid’ carbon dioxide. The critical point of carbon dioxide (not to be confused with the triple point, when all three phases exist in equilibrium) is at 304.25 K and 7.39 x 106 Pa. Above its critical point it behaves as a supercritical fluid, which expands to fill its container as if it were a gas but with a density as if it were a liquid.
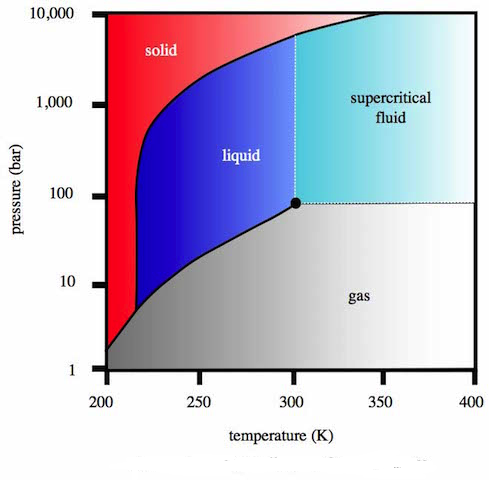
Phase diagram for carbon dioxide
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Environmental impact of some medications .
For short-answer questions see Environmental impact of some medications questions together with the worked answers on a separate page Environmental impact of some medications answers.
More resources
1. An excellent video from the International Atomic Energy Agency (IAEI), which looks at the methods of disposal of radioactive waste (both low-level and high-level) worldwide.
![]() Dealing with radioactive waste
Dealing with radioactive waste
2. A video from World Business Review on the management of waste solvent which looks at how solvents are cleaned and recycled in industry.
3. A general introduction to green chemistry by Martyn Poliakoff as part of the Nottinghamscience series.
4. For those with a real interest, the following article summarises some of the different ways in which oseltamivir has been synthesised: Recent progress in the synthesis of Tamiflu

 IB Docs (2) Team
IB Docs (2) Team 




















