4.3 Covalent structures (2)
This is the second part of 4.3 Covalent structures and looks at VSEPR theory to determine the shapes of simple molecules and ions. Since the underlying theory is the same it also includes 5 and 6 electrons pairs around the central atom which is on the Higher Level programme under 14.1.
.png)

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
.png) Understand:
Understand:
- Shapes of simple molecules and ions are determined by the repulsion of electron pairs according to Valence Shell Electron Pair Repulsion theory (VSEPR theory).
Apply your knowledge to:
- Use VSEPR theory to predict the electron domain geometry and the molecular geometry for species with two, three and four electron domains.
- Predict bond angles from molecular geometry and the presence of non-bonding pairs of electrons.
- Predict molecular polarity from bond polarity and molecular geometry.
 only: Deduce using VSEPR theory the electron domain geometry and molecular geometry with five and six electron domains and associated bond angles.
only: Deduce using VSEPR theory the electron domain geometry and molecular geometry with five and six electron domains and associated bond angles.
Relationships & vocabulary
Nature of science
Models are used by scientists as representations of the real world—VSEPR (the model of the shapes of simple molecules and ions) has been developed to explain observable properties.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 4 & 14: Chemical bonding & structure.
Vocabulary
| electron domain | tetrahedral | bent (or V-shaped) | trigonal planar | trigonal pyramid |
| octahedral | square planar | orthogonal | trigonal bipyramid | see-saw |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Part of sub-topic 4.3 is to predict the bond angles in species with two, three and four electrons domains. The classic example used for both Standard and Higher Level students is to compare the bond angles in methane, ammonia and water. It all seems highly logical. In methane all the four pairs of electrons (electron domains) are bonding pairs so repel each other equally to give a regular tetrahedron with bond angles of 109.5o. Ammonia contains one non-bonding pair which repels bonding pairs more than other bonding pairs so the bond angle decreases to about 108o in the trigonal pyramid molecule. The two non-bonding pairs in water cause even more repulsion so the bond angle in the bent molecule decreases further to about 104.5o. This can be found in all the text books and the theory perfectly matches the experimentally found bond angles.
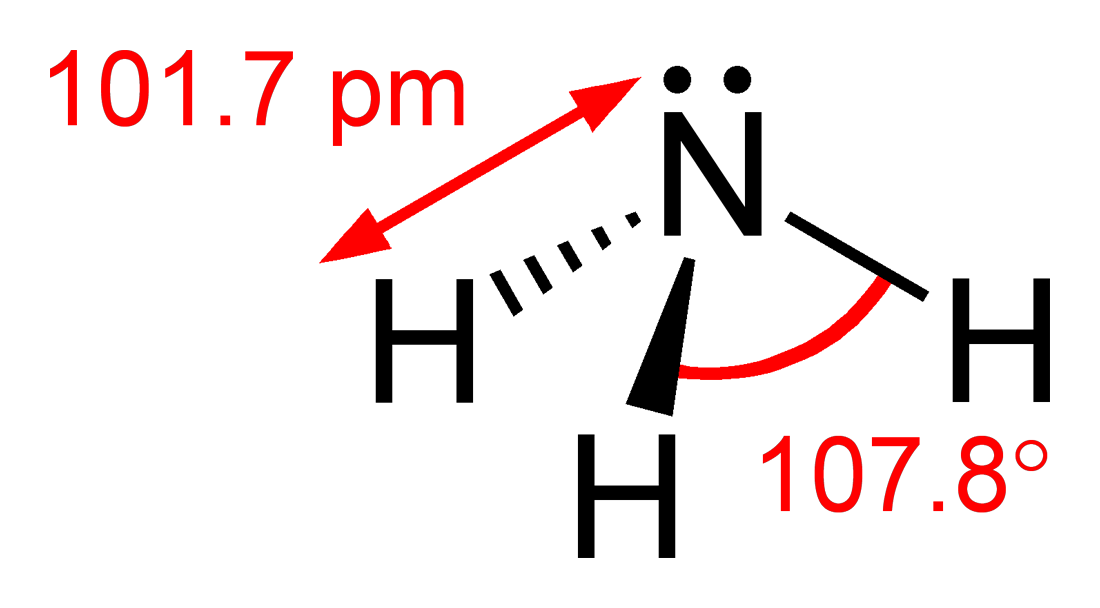

However this is yet another case of where the IB (and other pre-university courses) conveniently does not explore further as the logic appears flawed. Consider applying the same logic to phosphorus trichloride. Like ammonia there are three bonding pairs and only one non-bonding pair so one would expect the bond angle to be just less than 109.5o and more than 104.5o. In fact the bond angle is 100o. You might argue that it is the chlorine that perhaps makes the difference. So let's look at phosphine where the only difference with ammonia is that a phosphorus atom has replaced the nitrogen atom. The bond angle of 93.5o is even lower than phosphorus trichloride and considerably lower than the H-O-H bond angle in water where there are two non-bonding pairs.


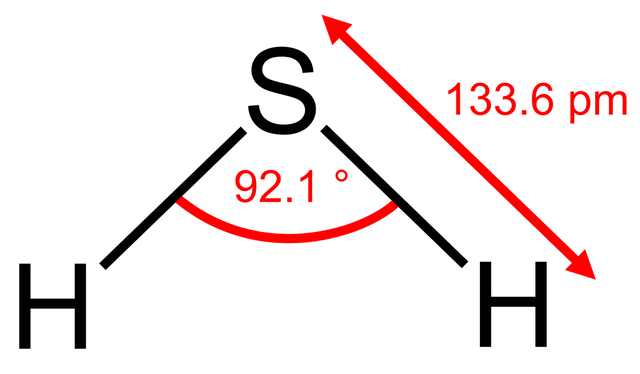
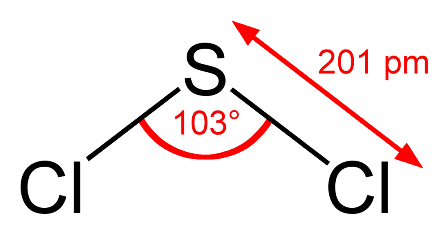
An astute student might argue that the bond angle also depends upon the period where the central atom is located since nitrogen and oxygen are in period 2 and phosphorus is in period 3. There is some truth in this. What would you expect the bond angle in hydrogen sulfide to be? On the simple theory it might be expected to be similar to water i.e. about 104.5o. If we now use what we know about phosphine then we might expect it to be a little less than 93.5o. Guess what - it is 92.1o - our new theory works! We could extend this and try to predict the bond angle for sulfur dichloride. Using our new logic we might expect it to be perhaps one or two degrees less than the 100o in phosphorus trichloride as there are now two non-bonding pairs of electrons? Wrong! It is 103o.
Clearly it is not nearly as simple as it is made out. Can you really be expected to predict these bond angles so that they agree with the experimentally determined values?
I don't think so!!
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Covalent structures (2).
For short-answer questions see Shapes & polarity questions.
More resources
1. A rather nice animation of shapes by a student - Ashley Jennings
2. A tutorial by Joey Smokey from Clark College Tutoring and Writing Center. This is just a lecture but the diagrams of the shapes are very clear.

 IB Docs (2) Team
IB Docs (2) Team 
































