20.2 Synthetic routes
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 20.2 Synthetic routes. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
 After studying this sub-topic you should be able to:
After studying this sub-topic you should be able to:
Understand:
- Synthesising organic compounds stems from readily available starting material via a series of separate steps. The basis of synthetic routes involves functional group interconversions.
- Synthetic design often involves retro-synthesis of organic compounds.
Apply your knowledge to:
- Deduce multi-step synthetic routes given starting reagents and the product(s).
Relationships & vocabulary
Nature of science
The thinking processes of organic chemists when designing syntheses can also involve retro-synthesis and the ability and imagination to think in a reverse-like manner. This is an important facet of the scientific method.
International-mindedness
Consider the importance of natural products (and the compounds that can be synthesised from them) to developing countries and their wider importance to the developed world.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 10 & 20 : Organic chemistry.
Vocabulary
retro-synthesis
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
This a rather strange sub-topic as basically it just makes links between other organic sub-topics and the reactions contained in them which is what a good teacher (and student) should be doing anyway! It does limit the number of stages to four which is ample as there are not actually that many reactions that are covered anyway. It can be useful to list these reactions:

What is significant, although perhaps not immediately obvious, is that many of these reactions only go one way. For example, alkenes react with hydrogen halides to give halogenoalkanes but the elimination of hydrogen halides from halogenoalkanes to give alkenes is not included in the syllabus. Similarly, esterification is on the core/AHL programme but saponification, the reverse process, is not. For this reason it is perhaps easier to show the possible reactions in a flow diagram:
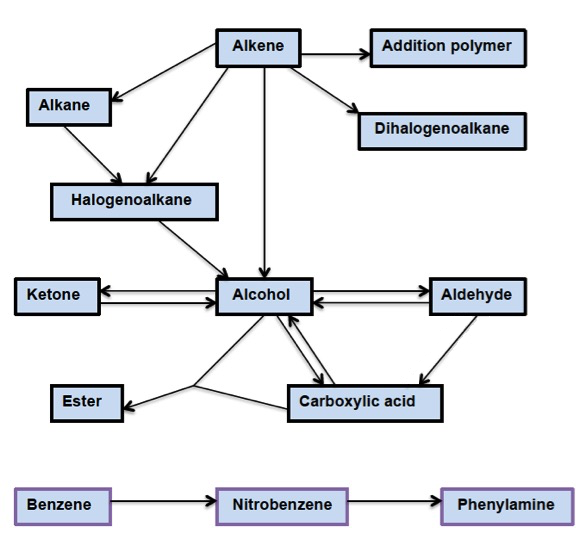
An analysis of this diagram does show that the range of syntheses that can be asked is quite limited. For example, the only synthesis involving arenes is the conversion of benzene into phenylamine via nitrobenzene and the route to an aldehyde, ketone or carboxylic acid is likely to come via the conversion of an alkene or halogenoalkane to an alcohol.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Synthetic routes.
For short-answer questions see Synthetic routes questions.
More resources
1. The separate reactions have already been covered in other sub-topics. Out of interest you may like to see how Bob Woodward achieved his multistage synthesis of chlorophyll in 1960. The full paper showing all 55 steps is surely one of the greatest of all chemistry research papers ever to be produced. A shortened version of the pathways for chlorophyll A is also available.
2. The problem with most other video resources on synthesis and retro-synthesis is that they use examples that involve many more reactions (e.g. elimination, Friedel Craft and Grignard reactions etc.) that are not covered on the IB programme so are not particularly helpful to IB students. This is also even true for videos on this topic on the past IB programme as the list of reactions has changed considerably and in the last programme the pathways were limited to just two steps. One set of notes that may be of some help is from A-levelchemistry.

 IB Docs (2) Team
IB Docs (2) Team 
















