20.1(3) Electrophilic substitution
 Learning outcomes
Learning outcomes
 After studying this sub-topic you should be able to:
After studying this sub-topic you should be able to:
Understand:
- The simplest arene (aromatic hydrocarbon compound) is benzene.
- Benzene has a delocalized structure of π bonds around its six-membered ring.
- Each carbon to carbon bond has a bond order of 1.5.
- Benzene undergoes electrophilic substitution reactions.
Apply your knowledge to:
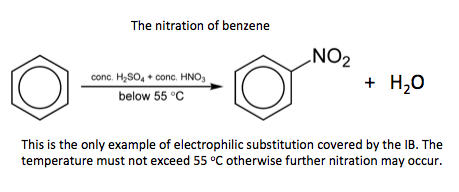
- Deduce the mechanism for the nitration of benzene using concentrated nitric acid and a catalyst of concentrated sulfuric acid.
Relationships & vocabulary
Nature of science
Organic chemical reactions involving functional group interconversions are among the key factors responsible for the progress made in the development and applications of scientific research.
International-mindedness
The misuse of alcohol is a serious problem in many countries and can have a detrimental impact both on their economies and on their social structures.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 10 & 20 : Organic chemistry.
Vocabulary
| electrophilic substitution | arene | nitration | nitronium ion |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
1. The structure of benzene
 The elucidation of the structure of benzene provides an interesting example of how science can progress. The structure of benzene had been puzzling chemists for years as none of the linear structures proposed could explain its properties. Friedrich Kekulé (1829-1896) was originally trained as an architect but switched to studying chemistry after hearing a lecture by Justus von Liebig. Kekulé was one of the originators of the theory of valency and the fact that carbon showed tetravalency. In 1865 he published a paper on the structure of benzene suggesting that it contained a hexagonal ring structure with alternate single and double carbon-carbon bonds. The evidence for this was mainly based on the fact that only one 1,2-disubstituted isomer of C6H4X2 existed as at that time bond lengths were not known.
The elucidation of the structure of benzene provides an interesting example of how science can progress. The structure of benzene had been puzzling chemists for years as none of the linear structures proposed could explain its properties. Friedrich Kekulé (1829-1896) was originally trained as an architect but switched to studying chemistry after hearing a lecture by Justus von Liebig. Kekulé was one of the originators of the theory of valency and the fact that carbon showed tetravalency. In 1865 he published a paper on the structure of benzene suggesting that it contained a hexagonal ring structure with alternate single and double carbon-carbon bonds. The evidence for this was mainly based on the fact that only one 1,2-disubstituted isomer of C6H4X2 existed as at that time bond lengths were not known.
Only when the 25th anniversary of the discovery was being celebrated in 1890 did Kekulé tell the story of how the discovery came to him through a dream. He dreamed that he was watching atoms twisting and entwining like snakes. One of the snakes caught hold of its own tail and it was this that gave him the inspiration to think of a closed ring structure. Whatever the truth of the dream story it was probably his training as an architect that gave him the ability to think of the structure in a different way. Later, in 1928, Linus Pauling introduced the idea of resonance in the structure. The model of benzene goes further than this. As a Higher Level student you need to know that each carbon atom in the benzene ring is sp2 hybridized and that the remaining electrons in the p orbitals form a delocalized π bond so that there is a volume of electron density above and below the planar ring which attracts electrophiles.
2. Why electrophilic substitution reactions of benzene are important.
The syllabus seems to limit the mechanism of electrophilic substitution reactions just to the nitration of benzene. This is a pity as once the idea of attack by an electrophile is understood benzene can be converted into many different classes of compounds by essentially the same reaction. In all of these reactions one of the hydrogen atoms in the benzene ring is substituted by an electrophile. The function of the H2SO4 in nitration and the halogen carrier, AlCl3, in chlorination, alkylation and acylation is to produce the electrophile. These catalysts do this by accepting a pair of electrons from the reacting species, HNO3, Cl2, RCl and ROCl, i.e. by functioning as Lewis acids.

Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Electrophilic addition & substitution reactions. Note that this quiz also includes questions on electrophilic addition as both form part of Topic 20.1.
For short-answer questions see Electrophilic substitution questions.
More resources
1. There are many videos available to explain electrophilic substitution - very few though limit themselves to just the nitration of benzene. The best way is to do it yourself (although as the use of benzene is not allowed in schools the usual experiment is the Preparation of 1,3-dinitrobenzene). Below is one where everything that is said is also written on the slides but nevertheless it is a reasonable account of the electrophilic substitution mechanism for the nitration of benzene.
2. This is perhaps a much better video, not just because it contains Homer Simpson and doughnuts (!), but also because it covers all electrophilic substitution reactions and also considers the effects of all the activating and deactivating groups. This is actually a bit beyond the current IB syllabus but still worth seeing (except that it sometimes uses non IUPAC names, e.g. toluene instead of methylbenzene).

 IB Docs (2) Team
IB Docs (2) Team 












