A.9 Condensation polymers
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option A - sub topic A.9. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)
 Learning outcomes
Learning outcomes
.png) After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- Condensation polymers are formed from monomers that contain at least two reactive functional groups .
- Possible inorganic products from condensation reactions include water, hydrogen chloride and ammonia .
- Kevlar® is an example of a polyamide with a strong and ordered structure. The hydrogen bonds formed between hydrogen and the oxygen and nitrogen atoms in the polymer can be broken by concentrated sulfuric acid.
Apply your knowledge to:
- Distinguish between addition and condensation polymerisation.
- Complete and describe equations showing the formation of condensation polymers.
- Deduce the structures of polyamides and polyesters from their respective monomers.
- Explain the strength of Kevlar® and its solubility in concentrated sulfuric acid.
Relationships & vocabulary
Nature of science
Will future civilisations classify our current era as the 'Polymer Age' as a way of continuing to describe how humans use science to manipulate matter as has been done with the Stone Age, Iron Age and Bronze Age in the past?
International-mindedness
What are the roles of science, economics and politics in the research and development of new polymers?
Vocabulary
| condensation polymerisation | polyester | polyamide | Kevlar® |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
It is worth looking at Kevlar in some detail, as one of the statements about Kevlar in the syllabus is a little confusing. Kevlar has been known since 1965. It was discovered by Stephanie Kwolek (1923 - 2014), an American chemist of Polish origins, whilst she was working for DuPont. Kevlar (polyparaphenylene terephthalamide) is a polyamide made from condensing 1,4-diaminobenzene with benzene-1,4-dicarbonyl chloride.
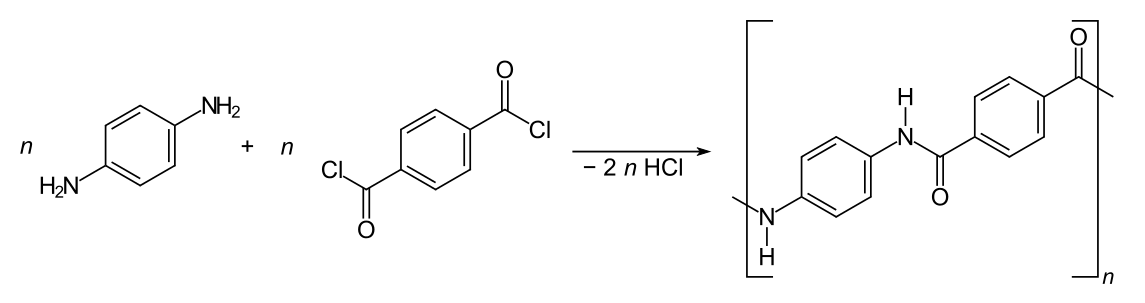
The formation of Kevlar from 1,4-diaminobenzene and 1,4-dicarbonyl chloride
It is actually quite expensive to make industrially as it needs to be kept in a solution of concentrated sulfuric acid during both its synthesis and when its fibres are being spun. This is to help prevent it forming into solid layers through cross-linking hydrogen bonds. Once the sulfuric acid is removed the three-dimensional cross-linked layers are then formed and it is this that gives Kevlar its immense strength. It is said to be more than five times stronger than steel although materials made of graphene are now even stronger. A recent paper reported that spiders sprayed with graphene nanoparticles produce a silk fibre, which is the strongest fibre ever measured.
One of the problems of polyamides like Kevlar is that they can be hydrolysed back to their monomers by acid solution. For this reason it is not a good idea to let car battery acid come into contact with materials made of nylon (such as climbing ropes). Kevlar is in fact more resistant to hydrolysis than nylon but concentrated sulfuric acid will break down the hydrogen bonding cross-links in Kevlar and severely weaken the structure. The syllabus states” The hydrogen bonds between O and N can be broken with the use of concentrated sulfuric acid”. This of course is not strictly true. The hydrogen bonds are not between the oxygen and nitrogen atoms but between the oxygen atoms of peptide linkages and the hydrogen atoms covalently bonded to the nitrogen atoms belonging to adjacent peptide linkages.

The structure of Kevlar (one repeating unit is shown in bold)
Kevlar has many uses. It is used to make the bulletproof vests employed by the military and police forces. Protective clothing such as motorcycle gear includes Kevlar to prevent cuts and abrasions in motorcycle accidents. It is also used to reinforce composite materials, in various musical instruments and to make ropes. There is good page of information on Kevlar produced by Bristol University in their series Molecule of the Month. Note that Kevlar can also act as a lyotropic liquid crystal (see A.4 Liquid crystals).
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Condensation polymers.
For short-answer questions see Condensation polymers questions together with the worked answers on a separate page Condensation polymers answers.
More resources
1. Jared Haymann from Elon University shows how nylon can be made in the lab.
2. A clear animation explanation of how polysters are made from the condensation reactions between dicarboxylic acids and diols.
3. A demonstration by DuPont on how gloves made from Kevlar are much more resistant to cuts than gloves made from cotton or leather.
![]() Demonstration of the strength of Kevlar
Demonstration of the strength of Kevlar

 IB Docs (2) Team
IB Docs (2) Team 












