4.5 Metallic bonding
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 4.5 Metallic bonding. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)

 Learning outcomes
Learning outcomes
.png) After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- Metallic bonds are formed by the electrostatic attraction between delocalized electrons and a lattice of cations (positive ions).
- The charge of the ions and the radius of the metal ion both affect the strength of a metallic bond.
- Alloys are usually comprised of more than one metal and have enhanced properties.
Apply your knowledge to:
- Explain electrical conductivity and malleability in metals.
- Explain trends in the melting points of metals.
- Explain the properties of alloys in terms of non-directional bonding.
Relationships & vocabulary
Nature of science
Natural phenomena can be explained by theories. For example, metals have different properties to covalent and ionic compounds because the "sea" of delocalized electrons forms non-directional bonds.
International-mindedness
One factor in determining national wealth is the availability of metal resources, and the means to extract them. This varies greatly from country to country. The demands for different metals change as technologies develop and in order to manage the supply of these finite resources careful strategies are required.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 4 & 14: Chemical bonding & structure.
Vocabulary
| alloy | brass | bronze | steel | delocalized |
| mobile | ductile | malleable | malleability |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
The not so rare ‘rare earth elements’
 Sub-topic 4.5 is very straightforward and it clearly states the trends should be limited to s and p block metals. There is a problem with the p block metal trends in group 14 as although tin has a smaller atomic radius than lead (1.40 x 10-10 m compared to 1.45 x 10-10 m according to the IB data booklet) it has a lower melting point (232 oC compared to 328 oC).
Sub-topic 4.5 is very straightforward and it clearly states the trends should be limited to s and p block metals. There is a problem with the p block metal trends in group 14 as although tin has a smaller atomic radius than lead (1.40 x 10-10 m compared to 1.45 x 10-10 m according to the IB data booklet) it has a lower melting point (232 oC compared to 328 oC).
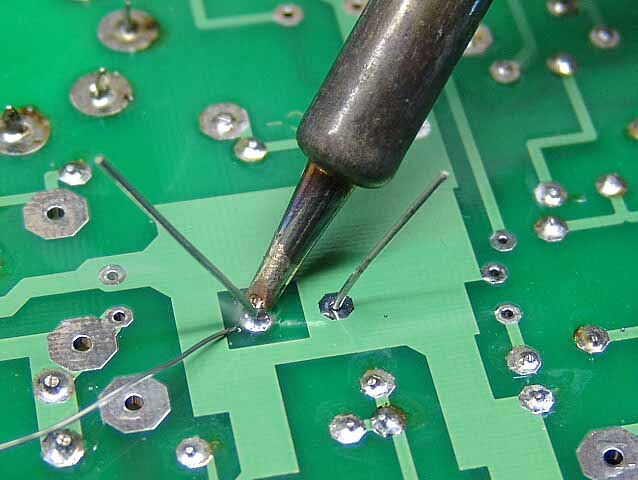
Although tin and lead form many different alloys, e.g. solder (shown being used in a circuit board in the image on the right), many of the best alloys come from combinations of transition metals. This is because transition elements in the same period have very similar atomic radii so inserting different metals into the crystal structure of a metal to form an alloy does not significantly disrupt the structure. For example, stainless steel contains iron (atomic radius 1.24 x 10-10 m) and up to 13% chromium (atomic radius 1.30 x 10-10 m) and may also contain nickel (1.17 x 10-10 m) and manganese (1.29 x 10-10 m).
The international-mindedness part of the syllabus refers to national wealth being related to the availability of metal resources, and the means to extract them. In sub-topic 3.1 The periodic table the syllabus also refers to the lanthanoids and actinoids. In recent years there has been much investment in extracting and purifying the so called rare earth metals. These are essentially fifteen lanthanoids together with scandium and yttrium. In fact they are not particularly rare, for example cerium has a similar abundance in the Earth’s crust to copper. However they do have many uses in modern technology.
.png)
Rare earth metals can be difficult and costly to mine and extract from their ores as they are often associated with radioactive material. In the 1990s the Chinese undercut many other sources and China is today the main supplier of rare earth elements. However in the past few years the Chinese have restricted the amount they export. This has caused the price of rare earth metals to rise and made the rest of the world seek new and alternative sources and also reopen mines that they had previously closed.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Metallic bonding.
For short-answer questions see Metallic bonding questions.
More resources
1. A video made by Richard Thornley specifically for this topic.
2. A fairly simplified video from two teachers, Jonathan Bergman and Aaron Sams which covers the basics of metallic bonding and why they conduct heat and electricity and are malleable.
3. Another video (for A level) by SnapRevise relating the properties of metals to metallic bonding.

 IB Docs (2) Team
IB Docs (2) Team 














