6.1 Collision theory & rates of reaction
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 6.1 Collision theory & rates of reaction. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
.png) Understand
Understand
- Reactions occur as a result of collisions between species that have sufficient energy and the correct orientation.
- Rate of reaction is expressed as the change in concentration of a particular reactant or product per unit time.
- The changes in concentration that occur during a reaction can be followed indirectly by monitoring changes in mass, volume or colour.
- The activation energy, Ea, is the minimum energy required by colliding molecules in order to react.
- A catalyst increases the rate of a chemical reaction by providing an alternative pathway with a lower activation energy, Ea, without itself being permanently changed chemically.
Apply your knowledge to:
- Describe kinetic theory in terms of the movement of particles whose average kinetic energy is proportional to temperature measured in Kelvin.
- Analyse graphical and numerical data from rate experiments.
- Explain the effects of temperature, pressure or concentration and particle size on the rate of reaction.
- Construct Maxwell–Boltzmann energy distribution curves to account for the probability of successful collisions leading to a reaction and factors affecting these, including the effect of a catalyst.
- Investigate rates of reaction experimentally and evaluate the results.
- Sketch and explain energy profiles with and without catalysts.
Relationships & vocabulary
Nature of science
Collision theory is a good example of the principle of Occam’s razor. This is used as a guide to developing collision theory based on current atomic models even though we cannot directly see reactions taking place at the molecular level.
International-mindedness
The catalytic action of CFCs is largely responsible for the depletion of stratospheric ozone and this is of particular concern in the polar regions. These chemicals are released from a variety of countries and sources, so global cooperation has been needed (e.g. The Montreal Protocol) to lower the amount of ozone depletion.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 6 & 16: Chemical kinetics.
Vocabulary
| kinetic theory | collision theory | rate of reaction | gradient | tangent |
| surface area | activation energy, Ea | Maxwell-Boltzmann curve | alternative pathway | catalyst |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Although many students know the factors that affect the rate of a chemical reaction, it is very noticeable when marking the answers to Paper 2 questions that when they are asked to define the term rate of reaction many of the answers show a woeful ignorance. The whole of Topic 6 depends upon understanding exactly what is meant by the term and how it can be applied and measured. The basic definition is that the rate of a chemical reaction is the increase in concentration of one of the products per unit time or the decrease in one of the reactants per unit time. The normal units are therefore mol dm-3 s-1. The problem is that the rate of a reaction changes as the reaction proceeds. This is because it depends upon the concentration of the reactants and this concentration is continually changing as the reactants are used up. To explain this it is useful to refer to graphs of change of concentration of products or reactants against time. The rate at time t can then be seen to be the gradient of the graph at that specified time. Probably the most useful rate is the initial rate as the initial concentration at time t = 0 will be known.
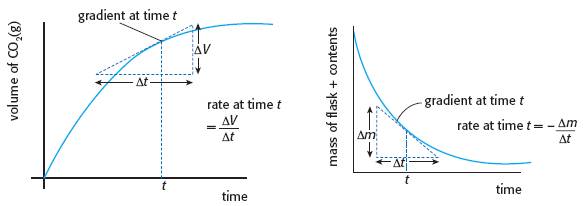
The diagram above shows the graphs obtained by following the rate of reaction between a metal carbonate and a mineral acid. In the first graph the volume of carbon dioxide evolved is plotted against time, in the second graph the loss in mass as the carbon dioxide is evolved from the reaction mixture is plotted against time. Practical details for these two methods are described in the practical Reaction rates.
Many books and teachers claim that increasing the temperature by 10 oC (10 K) will double the rate of the reaction. This is a useful 'rule of thumb' but that is all it is. In fact it works reasonably well when the temperature increases from 298 K to 308 K for typical reactions in which the activation energy is about 50 kJ mol-1 (50000 J mol-1). Higher Level students will be able to show this by substituting into the Arrhenius equation (see 16.2) and finding the values of the rate constant, k. Since the concentrations of the reactants remain constant and only the temperature is altered then the rate is directly proportional to the rate constant.
At 298 K: k298 = Ae-Ea/RT = Ae-50000/(8.314 x 298) = 1.72 x 10-9 A
At 308 K: k308 = Ae-Ea/RT = Ae-50000/(8.314 x 308) = 3.31 x 10-9 A
By increasing the temperature by 10 oC the rate constant has increased by 3.31 x 10-9 A / 1.72 x 10-9 A which amounts to 1.92 so effectively the rate has nearly doubled.
However if the activation energy is higher or lower than 50 kJ mol-1 then the ratio of the two rate constants changes considerably. For example, if the activation energy is 100 kJ mol-1 (100000 J mol-1) then by doing a similar calculation it can be shown that increasing the temperature from 298 K to 308 K increases the rate by 3.71 (i.e. nearly four times).
 Because changes in temperature can have such an effect on the rate of a reaction it is important to understand how to use a water bath (left) to maintain a constant temperature when designing an experiment where temperature is a controlled variable.
Because changes in temperature can have such an effect on the rate of a reaction it is important to understand how to use a water bath (left) to maintain a constant temperature when designing an experiment where temperature is a controlled variable.
Another misconception sometimes taught is why increasing the temperature increases the rate of a reaction. It is sometimes claimed that one of the major factors is due to an increase in the number of collisions and hence an increase in rate. It is true that there will be more collisions at higher temperatures but what is much more important is not the number of collisions but the number of successful collisions. Most collisions do not lead to a reaction and it has been estimated that the increased number of collisions due to increasing the temperature only accounts for about 10% of the increase in rate. The dominant factor affecting the rate is that the number of reactants particles that possess at least the minimum activation energy increases. As this number increases exponentially with temperature rise (see the Maxwell-Boltzmann curve) it is by far the most important factor.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Collision theory & rates of reaction.
For short-answer questions on collision theory see Collision theory questions.
For short-answer questions on rates of reaction see Rates of reaction questions.
More resources
1. An animated video to show the importance of collision theory by Richard Thornley.
2. You can find several videos showing the effect of surface area on rate e.g. exploding flour and lycopodium powder in a flame. Here is one which I'm sure you could do without leaving a mess on the floor!
3. A good video produced by Carleton University, Ottawa to illustrate that the enthalpy of reaction gives no information about how fast a reaction will proceed. It uses two examples with very similar enthalpy values (the rusting of iron and the Thermite reaction) that proceed at very different rates.
4. The IB includes the effects of concentration, temperature, surface area and a catalyst on the rate of a reaction but some reactions are also affected by light. Also, how do you measure the rate of very fast reactions in a laboratory? This video gives a good explanation of the technique of flash photolysis and provides a sophisticated example of the use of data logging.

 IB Docs (2) Team
IB Docs (2) Team 
















