13.2 Coloured complexes
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 13.2 Coloured complexes. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand:
Understand:
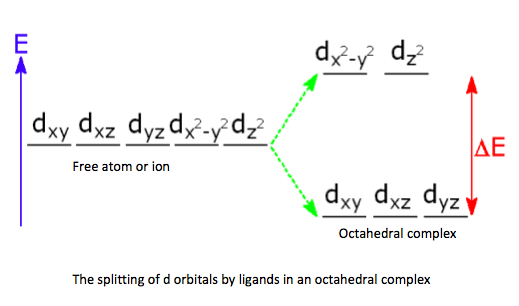
- Ligands cause the d sub-level in a complex ion to split into two sets of orbitals of different energy.
- Complexes of transition elements and ions are coloured because visible light is absorbed when an
electron is excited between the split d-orbitals. - The observed colour is the complementary colour to the colour of the absorbed light.
Apply your knowledge to:
- Explain that the colour of a transition metal ion complex is affected by the identity of the metal ion, the oxidation state of the metal and the identity of the ligand.
- Use the spectrochemical series to explain the effect of different ligands on the splitting of the d-orbitals in transition metal complexes and the colour observed.
Relationships & vocabulary
Nature of science
The different colours of transition metal complexes can be explained through the use of models and theories. The surrounding ligands cause the d-orbitals to split and the colour is the result of electronic transitions between the split d-orbitals.
Colour linked to symmetry cuts across disciplines and can be explored in the sciences, architecture, and the arts.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 3 & 13: Periodicity.
Vocabulary
| ligand | monodentate | polydentate | complex ion |
| complementary colour | spectrochemical series | EDTA |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Ligands
The definition of a ligand is that it is a neutral molecule or negative ion that donates one or more pairs of electrons to a metal atom or ion to form a coordination complex. Since it donates one or more pairs of electrons, by definition a ligand is also a Lewis base. The IB covers both monodentate ligands - that is ligands which only form just one coordinate bond with a transition metal - and polydentate ligands. Monodentate ligands include H2O, Cl–, CN–, NH3 and OH–. Polydentate (many teeth) ligands can form more than one coordinate bond with the transition metal atom or ion. They tend to bind quite strongly. As well as the three polydentate ligands (1,2-ethanediamine, ethanedioate and EDTA) that appear in Section 16 of the data booklet others appear on the syllabus in the options, for example porphyrins in Option B - Biochemistry and EDTA in the chelation of heavy metal ions in Option A - Materials. The concentration of transition metal complexes can be analyzed using visible spectroscopy. This can provide fertile ground for the Individual Scientific Investigation and for Extended Essays. EDTA, which is much used for chemical analysis, does have an IUPAC name but it is still routinely known by its common name EthyleneDiammineTetraAcetic acid or in its ionic form (see below) ethylenediaminetetraacetate. The non-bonding pairs of electrons on each of the two nitrogen atoms (blue in the diagram) and non-bonding pairs of electrons on each of the four oxygen atoms of the ethanoate (acetate) groups (red in the diagram) can all form coordinate bonds to a metal ion such as Cu2+.
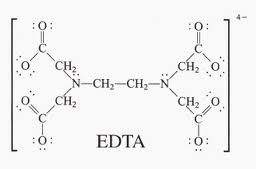
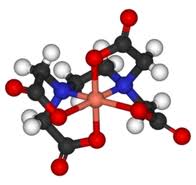
The free ligand ethylenediaminetetraacetate (left) and acting as a hexadentate ligand with Cu2+ (right).
Although probably not on the syllabus it is worth noting that some polydentate ligands can cause optical isomerism when they bond to transition metal ions. For example, when ethane-1.2-diamine (1,2-ethanediamine) reacts with the cobalt(III) ion two possible enantiomers can be formed.
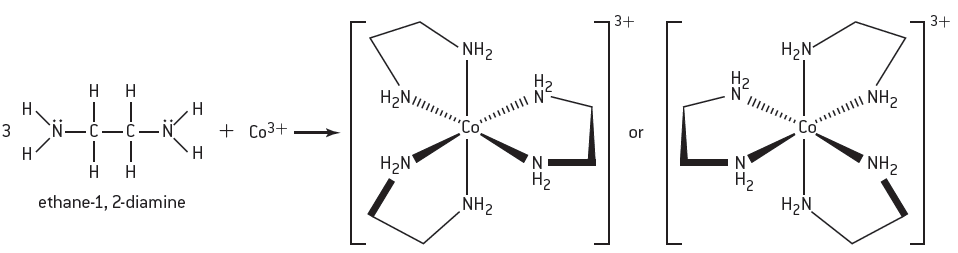
Three ethane-1,2-diamine bidentate ligands can form two different enantiomers with Co3+
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Coloured complexes .
For short-answer questions see Coloured complexes questions.
More resources
1. A good video showing how the complexes of vanadium with different oxidation states have different colours.
2. A video of a solution of copper sulfate reacting with ammonia solution. Hopefully you can get your teacher to demonstrate these reactions for you. (They work much more dramatically with concentrated ammonia solution alternating with water and concentrated hydrochloric acid - in a fume cupboard!).
3. A video produced by the Royal Society of Chemistry explaining visible spectroscopy.
4. An animated video showing how the d orbitals are split as ligands approach. The splitting is well explained for tetrahedral complexes (which are not on the syllabus) but not quite so well-explained for octahedral complexes.
![]() Crystal field theory - splitting of d orbitals
Crystal field theory - splitting of d orbitals

 IB Docs (2) Team
IB Docs (2) Team 










