8.1 Theories of acids & bases
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 8.1 Theories of acids & bases. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand:
Understand:
- Brønsted–Lowry acids are proton/H+ donors and Brønsted–Lowry bases are proton/H+ acceptors.
- The term amphiprotic refers to species that can act as both Brønsted–Lowry acids and bases.
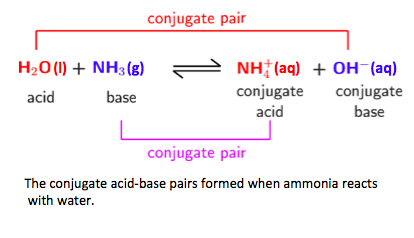
- A conjugate acid-base pair differ by a single proton.
Apply your knowledge to:
- Deduce the Brønsted–Lowry acids and bases in a chemical reaction.
- Deduce the conjugate acids or conjugate bases in a chemical reaction.
Relationships & vocabulary
Nature of science
The fact that both hydrochloric acid, HCl and prussic acid, HCN do not contain oxygen provides a good example of how theories such as Lavoisier's theory (that acids are oxides of non-metals with water) can be falsified and allow other theories to develop. This also provides an example of how theories are superseded.
Reference can also be made to the public understanding of science as terms such as 'the acid test' or 'litmus test' are often used in a non-scientific context.
International-mindedness
Acid–base theory has been developed by scientists from many different countries and its vocabulary has been influenced by their languages. For example, acidus means sour in Latin, alkali is derived from the Arabic word for calcined ashes and oxygene (thought by Lavoisier to be responsible for the acidic properties of a compound) means acid-forming in Greek.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 8 & 18: Acids & bases.
Vocabulary
| Arrhenius | BrØnsted-Lowry | proton donor | proton acceptor |
| conjugate acid | conjugate base | acid-base pair | amphiprotic |
| amphoteric |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Have some sympathy for Svante Arrhenius, the Swedish chemist who won the Nobel Prize for Chemistry in 1903. Sub-topic 8.1 is titled "Theories of acids and bases" and yet the only theory covered in the subsequent 'Understandings' is the BrØnsted-Lowry theory. Arrhenius's ionic theory is implied throughout all of the sub-topics 8.1 - 8.5 and in the AHL sub-topics in Topic 18 but it is not specifically mentioned. The Lewis theory of acids and bases is only covered at Higher Level. There are actually many theories of acids and bases. Early theories such as acids having a sour taste and Lavoisier's theory of an acid being the oxide of a non-metal and water are only of passing historical interest but Arrhenius's ionic theory is clearly still very important. This theory states that acids are substances that dissociate to give hydrogen ions in aqueous solution. Most of the other sub-topics in Topics 8 and 18 are in fact much more based on this definition than either the BrØnsted-Lowry or Lewis definitions so it seems strange that it has been left out of 8.1. For example, the pH scale, Ka and pKa calculations etc. are all concerned with the concentration of hydrogen ions in aqueous solution.
 Although Johannes BrØnsted and Thomas Lowry are usually lumped together they worked independently. BrØnsted (see right), who was a Dane, and Lowry from the UK, first proposed their theory in 1923. It considers the deprotonation of acids to form conjugate bases, or the protonation of bases to form conjugate acids. One of the biggest differences between the ionic theory and the BrØnsted-Lowry theory is when it is applied to water. Under the ionic theory pure water is neutral but under the BrØnsted-Lowry theory it can either be acidic or basic depending upon whether it is donating or accepting a proton. This property of water acting as either an acid or a base by donating or accepting a proton is described as amphiprotic. All amphiprotic substances can also be described as amphoteric (can act as either an acid or a base) but the reverse statement is not true as amphiprotic only applies to the BrØnsted-Lowry definition. Metals oxides, such as aluminium oxide, Al2O3, can react with acids or bases to forms salts and are therefore amphoteric but they cannot be described as amphiprotic as they are not able to donate a proton.
Although Johannes BrØnsted and Thomas Lowry are usually lumped together they worked independently. BrØnsted (see right), who was a Dane, and Lowry from the UK, first proposed their theory in 1923. It considers the deprotonation of acids to form conjugate bases, or the protonation of bases to form conjugate acids. One of the biggest differences between the ionic theory and the BrØnsted-Lowry theory is when it is applied to water. Under the ionic theory pure water is neutral but under the BrØnsted-Lowry theory it can either be acidic or basic depending upon whether it is donating or accepting a proton. This property of water acting as either an acid or a base by donating or accepting a proton is described as amphiprotic. All amphiprotic substances can also be described as amphoteric (can act as either an acid or a base) but the reverse statement is not true as amphiprotic only applies to the BrØnsted-Lowry definition. Metals oxides, such as aluminium oxide, Al2O3, can react with acids or bases to forms salts and are therefore amphoteric but they cannot be described as amphiprotic as they are not able to donate a proton.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Theories of acids & bases.
For short-answer questions see Theories of acids & bases questions.
More resources
1. A tutorial on the different theories of acids and bases which is at about the right level for IB Chemistry
2. A very straightforward description of acid-base conjugate pairs from chemistNATE reinforcing BrØnsted-Lowry theory with examples.
BrØnsted-Lowry acid-base pairs ![]()

 IB Docs (2) Team
IB Docs (2) Team 










