2.2 Electron configuration
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 2.2 Electron configuration. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
Understand:
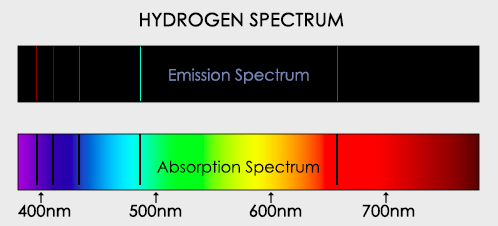
 When electrons that have been excited by gaining energy return to a lower energy level in an atom they emit photons and produce an emission spectrum.
When electrons that have been excited by gaining energy return to a lower energy level in an atom they emit photons and produce an emission spectrum.- Evidence for the existence of electrons in discrete energy levels comes from the line emission spectrum of hydrogen, in which the lines converge at higher energies.
- The main energy level (or shell) is identified by an integer number, n, and can hold a maximum number of electrons equal to 2n2.
- The main energy levels can be split into s, p, d and f sub-levels with successively higher energies.
- Sub-levels contain a fixed number of orbitals. An orbital is a volume of space where there is a high probability of finding an electron.
- Each orbital has a defined energy state for a given electronic configuration and chemical environment.
- Each orbital can contain a maximum of two electrons each with opposite spin.
Apply your knowledge to:
- Describe the relationship between colour, wavelength, frequency and energy across the electromagnetic spectrum.
- Distinguish between a continuous spectrum and a line spectrum.
- Describe the emission spectrum of the hydrogen atom, including the relationships between the lines and energy transitions to the first, second and third energy levels.
- Recognize the shape of an s atomic orbital and the px, py and pz atomic orbitals.
- Apply the Aufbau principle, Hund’s rule and the Pauli exclusion principle to deduce electron configurations for atoms and ions up to Z = 36.
Relationships & vocabulary
Nature of Science
An example of the improvement in apparatus leading to developments in science is the use of electric and magnetic fields in Thomson's cathode ray tubes.
Quantum mechanics provides the basis for current models of the atom superseding the Bohr model.
Natural phenomena can be explained by theories e.g. line spectra can be explained by the Bohr model of the atom.
International-mindedness
CERN (The European Organization for Nuclear Research) in Switzerland is run by 20 European member states and involves scientists from many other countries. Its particle accelerators and detectors are used to study and research the fundamental constituents of matter.
For examples and more links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 2 & 12: The nuclear atom.
Vocabulary
| emission spectrum | discrete | continuous |
| s, p, d and f orbitals | Aufbau principle | Hund's rules |
| Pauli exclusion principle | electron configuration | Heisenberg's uncertainty principle |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about

Practically the diagram on the left can be used to determine the order in which orbitals are filled by electrons. It is known either as the Madelung rule or as the Klechkowski rule although in practice many people know it simply as the n +1 rule. The rule does not actually work for chromium and copper. The order can be more logically deduced by looking at graphs of ionization energies or from the position of elements in the periodic table but what is the theory behind it?
Under 'Applications and skills' in sub-topic 2.2 it states “Application of the Aufbau principle, Hund's rules and the Pauli exclusion principle to write electron configurations for atoms and ions up to Z = 36.” Technically knowledge of quantum numbers is required to do this although quantum numbers are not on the IB programme.
Pauli’s exclusion principle was formulated by Wolfgang Pauli in 1925. It applies to all particles collectively known as fermions which include protons and neutrons as well as electrons. Fermions are particles with half spin and the value of the spin quantum number can either be + ½ or – ½. At this level we restrict it to just electrons and Pauli’s exclusion principle states that no two electrons within an atom can possess the same four quantum numbers. The principle quantum number (n) refers to the main energy level and can have values 1, 2, 3….. The azimuthal quantum number (l) is related to angular momentum and has the values 0 to n −1. It determines the type of orbital or sub energy level; s (l=0), p (l=1), d (l=2) and f (l=3). Thus when n = 1 there are only s orbitals as l can only have the value of zero whereas when n = 3 there are s, p and d orbitals. The magnetic quantum number (m) has values –l, -(l−1)...0...(l+1), l and determines the number of orbitals within a sub-level. For example, when l = 1 there are three values −1, 0, and +1 which explains why there are three p orbitals (px, py and pz). Finally there is the spin quantum number (s) which can have the values of + ½ or – ½ which explains why each orbital can contain only two electrons. Pauli’s exclusion principle is therefore the basis of the Aufbau principle. Hund’s rules are required to state that electrons fill up orbitals of the same type singly with the same spin before they spin pair. This explains why there is a drop in first ionization energies between nitrogen (1s22s22px12py12pz1) and oxygen (1s22s22px22py12pz1) as it is energetically easier to remove the spin paired electron in oxygen. Hund's rules can also be used to explain the chromium and copper exceptions.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Electron configuration.
For short-answer questions see Electron configuration questions.
More resources
1. A really nice video showing how all the orbitals build up around the nucleus to give the configuration for transition metals. It actually says it is for scandium but since no electrons are added it could be for any element between scandium and zinc.
2. Maybe instead you prefer your orbitals to be shown using balloons? If so take a look at this Brightstone video on atomic orbitals.

 IB Docs (2) Team
IB Docs (2) Team 


























