D.3 Opiates
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option D - sub topic D.3. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand:
Understand:
- The chemical structure and solubility of a drug in water and lipids affects its ability to cross the blood–brain barrier.
- Opiates are derived from the opium poppy and are natural narcotic analgesics.
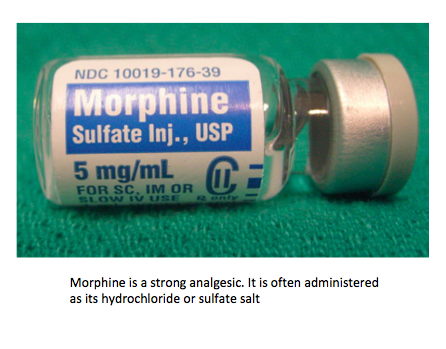
- Morphine, codeine and diamorphine (heroin) are examples of strong analgesics. Strong analgesics function by temporarily bonding to receptor sites in the brain. They prevent the transmission of pain impulses without depressing the central nervous system.
- The medical use and the addictive properties of opiate compounds are related to the presence of opioid receptors in the brain.
Apply your knowledge to:
- Explain the synthesis of codeine and diamorphine from morphine.
- Describe and explain the use of strong analgesics.
- Compare the structures of morphine, codeine and diamorphine (heroin).
- Discuss the advantages and disadvantages of using morphine and its derivatives as strong analgesics.
- Discuss the side effects and addiction to opiate compounds.
- Use their chemical structure and solubility to explain the increased potency of diamorphine compared to morphine.
Relationships & vocabulary
Nature of science
Opium has been used as a painkiller for thousands of years. Chemists have adapted this natural substance to produce semi-synthetic derivatives (e.g. diamorphine) with similar properties.International-mindedness
Many illegal drugs, including opiates, are grown and/or produced in a relatively small number of countries and then trafficked and sold worldwide.
There are different cultural and economic views on the production and sale of opiates around the world.
Vocabulary
| morphine | diamorphine (heroin) | codeine |
| opiate | lipid | semi-synthetic |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Some care needs to be taken with some of the statements made in the subject guide about morphine and its derivatives.
In the Nature of Science section for D.3 it states
“Data and its subsequent relationships—opium and its many derivatives have been used as a painkiller in a variety of forms for thousands of years. One of these derivatives is diamorphine“
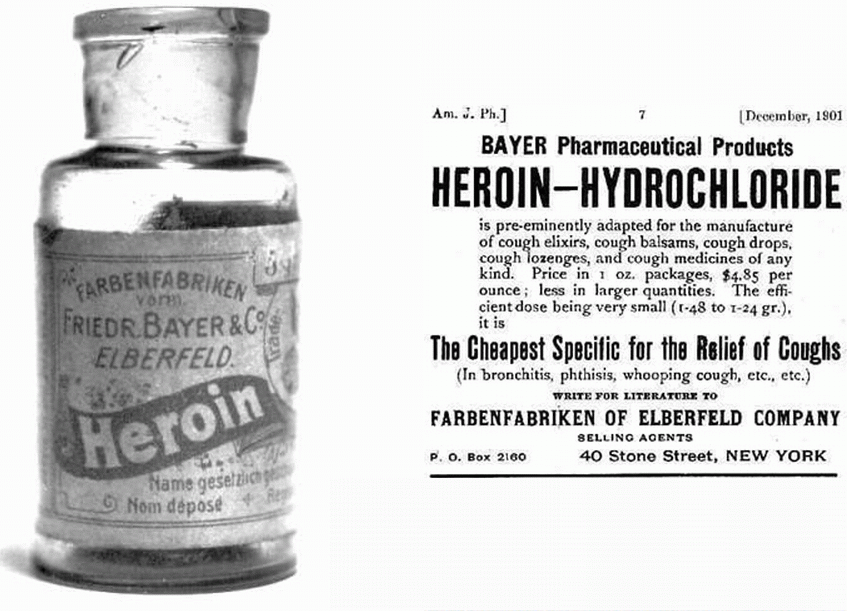
This implies to me that diamorphine (heroin) has been known and used for ‘thousands of years’. The opium poppy (Papaver somniferum) does naturally contain over 40 different alkaloids. Most prominent amongst these is morphine (about 12 - 21%) with the second most prominent being codeine (0.5 - 2.5%). Morphine was first identified chemically in 1805 by Friedrich Sertürner (1783-1841) and it was used as a pain-killer in the American War of Independence. Codeine was isolated from opium three decades later in 1832 by Pierre Robiquet (1780 – 1840), a French chemist. However diamorphine (heroin) is semi-synthetic and is not present naturally in the opium poppy. It was first synthesised from morphine in the same decade (the 1890s) and by the same company that first synthesised aspirin – the Bayer Company in Germany. It certainly has not been known for ‘thousands of years’.
The first statement in the ‘Application and skills’ section is “Explanation of the synthesis of codeine and diamorphine from morphine. Codeine of course does not have to be synthesised from morphine as it does occur naturally however because it only occurs naturally in relatively small mounts much that is produced industrially is synthesised from morphine. I have included a good video from the Royal Society of Chemistry in the ‘other resources’ section below that gives a very thorough explanation as to how the phenol group is substituted for an ether group. Essentially it involves using iodomethane as the reagent in a nucleophilic substitution reaction. The synthesis of diamorphine from heroin is very similar to the synthesis of aspirin from salicylic acid as ethanoic anhydride is used in both cases in preference to just ethanoic acid, as it is a better nucleophile. The only major difference is that the synthesis of diamorphine is an example of diesterification rather than monoesterification.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Opiates.
For short-answer questions see Opiates questions together with the worked answers on a separate page Opiates answers.
More resources
1. An excellent video from the Royal Society of Chemistry explaining how codeine can be semi-synthesised from morphine by nucleophilic substitution.
![]() Synthesis of codeine from morphine
Synthesis of codeine from morphine
2. Another good video on morphine and heroin from Nottingham University which also explains its chemistry.

 IB Docs (2) Team
IB Docs (2) Team 














