Culture of Chemistry (2)
The Culture of Chemistry quizzes illustrate some of the Nature of Science aspects of the course. They aim to widen your general knowledge about our subject and give you some genuine examples of how chemistry works practically both now and in the past in everyday life as well as theoretically. Each quiz consists of ten multiple choice questions. Full answers and explanations are provided to hopefully increase your enjoyment and love of chemistry as well as stimulate your interest to perhaps do some further research.
Introduction
This is the second of the Culture of Chemistry quizzes. The quizzes have several aims. Essentially they illustrate some of the Nature of Science aspects of the course. They may also be helpful for your study of Theory of Knowledge as culture is one of the twelve TOK concepts. The questions are unlikely to contain content that will be examined as such in the May/November examination sessions but they aim to widen your general knowledge about our subject and give you some genuine examples of how chemistry works practically both now and in the past in everyday life as well as theoretically. They are meant to be fun and perhaps in these current Covid times also provide a little bit of light relief. I would not expect you to know many of the answers so the score you get is irrelevant but I have provided fulsome answers and explanations to hopefully increase your enjoyment and love of chemistry as well as stimulate your interest to perhaps do some further research. Some of the questions may provide you with the spark of an idea that you could develop for your individual scientific investigation (IA) or extended essay.
Culture of Chemistry (2) Quiz
What is the H−O−H bond angle in the hydronium ion, H3O+?
A good scientific theory should enable scientists to make predictions. According to VSEPR theory the electron domain geometry around the central oxygen atom should be tetrahedral made up of three bonding pairs and one non-bonding pair of electrons so that the actual shape of the ion is trigonal pyramidal. Apart from the overall positive charge this is the same as ammonia. The prediction therefore is that the bond angle will be less than 109.5o and about the same as ammonia which is 107o. However information from infrared studies have shown that the bond angle in the gaseous phase is actually 113.6o which brings into question the validity of VSEPR theory.
When graphs of the 1st ionization energies are plotted for the elements (as in the one shown below) it is usual to connect the points rather than draw the line of best fit. Which is the best explanation for this?
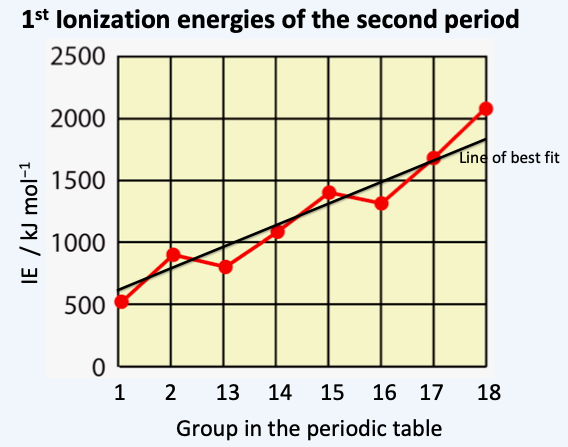
A continuous variable is a variable whose value is obtained by measuring whereas a discrete variable is a variable whose value is obtained by counting. Continuous variables (which are used most often in chemistry) can have an infinite number of possible values between two intervals, for example the volume of a gas evolved during a reaction. Discrete variables have separate indivisible categories so that no value can exist between neighbouring categories. This means that you cannot interpolate between the intervals so a best fit line is meaningless. Note that the x-axis is actually linear if atomic number (rather than group) is used as the value increases by one for each interval.
Who discovered the first commercially successful synthetic thermoplastic polymer while working for DuPont and then took his own life by cyanide poisoning two years later?
The first synthetic thermoplastic polymer was a polyamide called nylon discovered in 1935 by Wallace Carothers. It was made by reacting hexanedioic acid, HOOC-(CH2)4-COOH with hexane1,6-diamine, H2N-(CH2)6)-NH2. Carothers suffered from severe depression and died in 1937 just before nylon became commercially available as a substitute for silk.

Wallace Carothers (1896-1937)
What is the origin of the term “copper-bottomed investment” which can be used to describe a venture, plan or investment that is safe and is certain to be successful?
By the late 18th century the bottom of some wooden merchant ships were lined with copper. It helped to prevent attack by worms and barnacles as the copper acted both as a barrier and as a poison. Before this practice valuable cargo was sometimes lost through ships sinking. These ships were often funded by a group of investors. Placing their money in ships that had copper bottoms was seen as a much more reliable investment.
What is the chemical formula for Epsom salts?
Many chemicals that have been known for a long time have common names. CuSO4.5H2O is blue vitriol, Na2CO3.10H2O is washing soda, NaNO3 is Chile saltpetre and MgSO4.7H2O is Epsom salts. Now the IUPAC systematic way of naming compounds is used by scientists (e.g. magnesium sulfate heptahydrate) but their common names are still in use elsewhere. Epsom salts is named after a town in the UK where it occurs in the mineral water. It can be added to bath water to aid relaxation but taken internally it can cause diarrhoea. “If the bottom is falling out of your world, take Epsom salts and the world will fall out of your bottom”.
In 1857 the author Charles Dickens wrote,
"Within the course of the last two years .. a treasure has been divined, unearthed and brought to light ... what do you think of a metal as white as silver, as unalterable as gold, as easily melted as copper, as tough as iron, which is malleable, ductile, and with the singular quality of being lighter that glass? Such a metal does exist and that in considerable quantities on the surface of the globe.“
Which metal was he referring to?
In 1865 Jules Verne described aluminium in a similar fashion (an early example of almost plagiarism?) as he chose it as the material for his rocket in his novel “De la terre á la lune” (From the Earth to the Moon).
"This valuable metal possesses the whiteness of silver, the indestructibility of gold, the tenacity of iron, the fusibility of copper, the lightness of glass. It is easily wrought, is very widely distributed, forming the base of most of the rocks, is three times lighter than iron, and seems to have been created for the express purpose of furnishing us with the material for our projectile."
Which is the only element to have been discovered on a star before it was found on Earth?
Helium was discovered in 1886 in the spectral lines from the sun by the French astronomer Pierre Janssen and independently by the British astronomer Norman Lockyer who named it helium after Helios, the Greek god of the sun. It was confirmed as being present on Earth in 1895 when it was found emanating from an ore of uranium.
The German Messerschmitt 163 is the only rocket aeroplane to have been involved in combat. It was used in the later stages of World War II. The fuel was a mixture of C-stoff (hydrazine, N2H4, in methanol) and T-stoff (hydrogen peroxide). The products of the combustion are nitrogen and water. Which factors are necessary for a good rocket fuel?
I. The reaction must be fast
II. The reaction must be highly exothermic
III. The products must have low molar masses
The reaction must be able to release a large amount of energy in a short space of time and the exhaust velocity must be high to provide the thrust which means the molar mass of the products should be as low as possible.
Which group of toxic chemicals was widely used in electrical capacitors and transformers before a worldwide ban in 2001 but still persists in the oceans causing high levels in fish and other marine life?
PCBs are persistent organic pollutants as they are very stable chemically with a long half-life so they persist in the environment. PCBs cause skin damage, liver disease, cancer and can affect cognitive ability in children.
What is the concentration of 2N sulfuric acid, H2SO4(aq), when expressed in mol dm−3
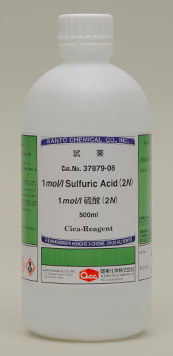 This is an example of the language of chemistry. Normality measures concentration in gram equivalent weight per litre. Equivalent weight is the mass of a given substance which will combine with or displace a fixed quantity of another substance. Since sulfuric acid is a diprotic acid it contains two equivalents so a 1 mol dm−3 solution of H2SO4 has a concentration of 2N. Although still used in some industries, equivalent weights and normality ceased being used in schools in most countries in the 1970s. IUPAC does not recommend the use of normality as the definition of the equivalence factor may vary depending on the type of chemical reaction involved.
This is an example of the language of chemistry. Normality measures concentration in gram equivalent weight per litre. Equivalent weight is the mass of a given substance which will combine with or displace a fixed quantity of another substance. Since sulfuric acid is a diprotic acid it contains two equivalents so a 1 mol dm−3 solution of H2SO4 has a concentration of 2N. Although still used in some industries, equivalent weights and normality ceased being used in schools in most countries in the 1970s. IUPAC does not recommend the use of normality as the definition of the equivalence factor may vary depending on the type of chemical reaction involved.

 IB Docs (2) Team
IB Docs (2) Team