10.1 Fundamentals of organic chemistry
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 10.1 Fundamentals of organic chemistry. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand
Understand
- A series of compounds of the same class that have the same general formula, but differ from each other by a common structural unit, is known as an homologous series.
- Either the full or the condensed format can be used to represent structural formulas.
- Compounds with the same molecular formula but with a different arrangement of atoms are known as structural isomers.
- A functional groups is the reactive part of a molecule.
- Saturated compounds only contain single bonds. Unsaturated compounds contain double and/or triple bonds.
- Benzene is an aromatic, unsaturated hydrocarbon represented by
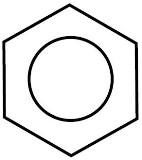 .
.
Apply your knowledge to:
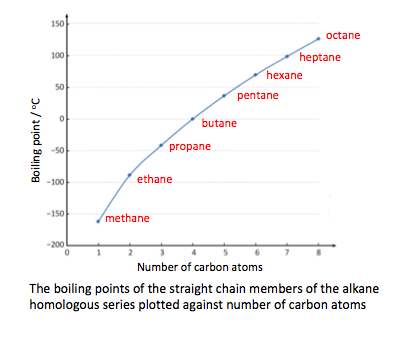
- Explain the trends in the boiling points of members of a homologous series.
- Distinguish between empirical, molecular and structural formulas.
- Identify the following different classes: alkanes, alkenes, alkynes, halogenoalkanes, alcohols, ethers, aldehydes, ketones, esters, carboxylic acids, amines, amides, nitriles and arenes.
- Identify typical functional groups in molecules e.g. phenyl, hydroxyl, carbonyl, carboxyl, carboxamide, aldehyde, ester, ether, amine, nitrile, alkyl, alkenyl and alkynyl.
- Construct real or virtual 3-D models of organic molecules.
- Apply the IUPAC rules to name straight-chain and branched-chain isomers.
- Identify primary, secondary and tertiary carbon atoms in halogenoalkanes and alcohols and primary, secondary and tertiary nitrogen atoms in amines.
- Use physical and chemical evidence to discuss the structure of benzene.
Relationships & vocabulary
Nature of science
Organic chemistry contains many examples of serendipity in the discovery of compounds and their uses, e.g. penicillin, teflon and superglue.
Organic chemistry also provides many instances where ethical implications are important. For example, drugs, pesticides and additives can all have positive and negative effects on humans, other animal species and the environment.
International-mindedness
Global policies and economic development are often shaped by supply and demand. This is especially evident with the world's reserves of oil as relatively few countries have control over its extraction and supply.
The performance of the fuel used in cars and aircraft is measured by its octane number or octane rating. Octane ratings can differ quite widely from country to country. This is further complicated by the fact that different countries use different ways to express the values.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 10 & 20 : Organic chemistry.
Vocabulary
| homologous series | structural isomer | skeletal formula | IUPAC |
| alkane, alkene, alkyne, alcohol, ether | aldehyde, ketone, carboxylic acid | halogenoalkane, amine, ester, nitrile, arene. | hydroxyl, carboxyl |
| cyclic and non-cyclic | primary, secondary, tertiary |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
‘Fundamentals of organic chemistry' is rather a strange name for this first sub-topic. There are no explanations given as to why organic chemistry is important or why more compounds of carbon are known than the sum of all the compounds of all the other elements present in the periodic table. There is also no explanation as to why the chemistry of carbon is called ‘organic’ chemistry. Essentially this sub-topic lists several classes of compounds and you are required to understand the difference between 'class' and 'functional group' and be able to relate properties such as boiling points to members of each class. The classes specified are alkanes, alkenes, alkynes, halogenoalkanes, alcohols, ethers, aldehydes, ketones, esters, carboxylic acids, amines, amides, nitriles and arenes. You are also expected to be able to deduce and/or recognise the names and structures for compounds containing up to six carbon atoms in these classes and to make 3-D models of these compounds. You should also understand the terms homologous series and structural isomers and know the difference between saturated and unsaturated and be able to discuss the evidence for the structure of benzene.
Naming organic compounds can cause some problems even when the compounds are relatively simple. Generally the IB follows the preferred IUPAC naming system. So that CH3CH(OH)CH3 is called propan-2-ol by the IB when referred to in the syllabus or in examination questions although 2-propanol or isopropyl alcohol are both perfectly acceptable alternative names and would be accepted by the IB in a student's answer to a question (although isopropyl alcohol would not be accepted if the questions asked specifically for the IUPAC name).
You should be familiar with the various ways of representing organic molecules. These include full structural formulas showing the bonds between all the atoms and condensed structural formulas. You should also be able to use and interpret the various different ways of modelling molecules, including ball and stick and space-filling models. To exemplify this, the following are some of the different ways of representing propan-2-ol. Note that the last two are skeletal or part-skeletal structures and you should also know these for the IB. Because a line represents two carbon atoms in a skeletal structure you must show all the hydrogen atoms when they are giving the full structural formula of a compound.
.png)
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Fundamentals of organic chemistry.
For short-answer questions see Fundamentals of organic chemistry questions (1) and Fundamentals of organic chemistry questions (2).
More resources
1. There are many rather boring videos on YouTube on naming organic compounds. It is not really a topic to make an exciting video but Mark Rosengarten seems to have managed it with this video by setting it all to music. You might enjoy it.
2. A video by Richard Thornley showing how the different strengths of intermolecular forces of attraction affect volatility and solubility in water for several different classes of organic compounds.

 IB Docs (2) Team
IB Docs (2) Team 


































