5.2 Hess's Law
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 5.2 Hess's law. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand
Understand
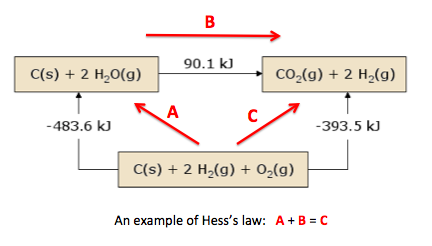
- Hess's Law states that the enthalpy change for a reaction that is carried out in a series of steps is equal to the sum of the enthalpy changes for the individual steps, i.e. it is independent of the reaction pathway.
Apply your knowledge to:
- Apply Hess’s Law to calculate enthalpy changes.
- Calculate the value of ΔH⦵ for a reaction using data for ΔHf⦵ values.
- Determine the enthalpy change of a reaction that is the sum of multiple reactions with known enthalpy changes.
Relationships & vocabulary
Nature of science
Testing hypotheses. Hess's Law states that if the same products are formed from the same initial reactants then the energy change should be the same regardless of the number of steps. This is an example of where a hypothesis based on the law of conservation of energy and on atomic theory can be tested experimentally.
International-mindedness
There is a rather strange reference in the syllabus about recycling. It is not clear why this is under a sub-topic on Hess's Law but it states that although recycling materials is often an effective means of reducing the environmental impact of production, its efficiency in energy terms can vary in different countries.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 5 & 15: Energetics/thermochemistry.
Vocabulary
| Hess's Law | Law of conservation of energy | First law of thermodynamics | Enthalpy cycle |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Hess’s Law is named after Germain Henri Hess (1802 – 1850) a Swiss national who moved with his family to Russia at the age of 3. He qualified as a doctor but later became a professor of chemistry at the St Petersburg Technical Institute. He was interested in minerals and analyzed one particular ore of silver telluride, Ag2Te, which is now called hessite in his honour.


Germain Hess Hessite
Hess’s Law states that the total energy change in a chemical reaction depends only upon the initial and final states and is independent of the reaction pathway. It used to be known as the Law of Constant Heat Summation and is in fact another way of stating the Law of Conservation of Energy.
So how can Hess’s Law be applied?
There are basically three different methods that can be used to solve Hess’s Law problems:
- Energy cycle
- Enthalpy level diagram
- Simultaneous equations
So which is the best method to train students to use?
Energy cycle, enthalpy level diagram or simultaneous equations?
Take as an example the following question.
The enthalpy change for the dimerization of two mol of nitrogen dioxide to form one mol of dinitrogen tetroxide is – 57 kJ.
2NO2(g) → N2O4(g) ∆H![]() = – 57 kJ
= – 57 kJ
The enthalpy change for the formation of one mol of NO2(g) from its elements is + 33 kJ
½ N2(g) + O2(g) → NO2(g) ∆H![]() = + 33 kJ
= + 33 kJ
What is the enthalpy change for the formation of one mol of dinitrogen tetroxide from its elements?
N2(g) + 2O2(g) → N2O4(g)
1. Solution using simultaneous equations.
To get N2 on the left hand side start with the second equation and double it:
N2(g) + 2O2(g) → 2NO2(g) ∆H![]() = + 66 kJ
= + 66 kJ
To get rid of 2NO2 and to get N2O4 on the right hand side add the first equation:
N2(g) + 2O2(g) → N2O4(g) ∆H![]() = + 66 + (– 57) = + 9 kJ
= + 66 + (– 57) = + 9 kJ
Easy - but it does not really involve any chemistry.
2. Solution using an enthalpy diagram.
The problem is that you need to put the reactants and products in the correct places on the diagram.

Personally I do not find it very obvious from the information given even though there is no need to include the oxygen (as you do not know the answer beforehand) and students certainly do not find this easy.
Once the diagram has been drawn correctly it is easy to see that x is positive and is the difference in value between 66 and 57 kJ.
3. Solution using an energy cycle.
Use the idea of A going directly to B or via C. In this case A is N2, B is N2O4 and C is NO2.
In chemistry terms nitrogen can either be oxidized directly to N2O4 or first to NO2 which then dimerizes
to N2O4. (Again there is no need to include the oxygen in the cycle.)

From the diagram (i.e. applying Hess’s Law) it is easy to see that x = (2 x 33) + (– 57) = + 9 kJ
All three methods are correct but I think the energy cycle method is the easiest, the most elegant and demonstrates the best understanding of chemistry.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Hess's Law.
For short-answer questions see Hess's Law questions.
More resources
1. A tutorial which explains how to solve Hess's Law problems just by using simultaneous equations. It works, but in my view (see above), by ignoring the use of energy cycles it does not show any real understanding.
2. Students teaching students. It could be worth showing this to students as they might appreciate it. Although quite thorough it does use three separate reactions simply to verify Hess's Law rather than using it to find out a value that cannot be determined directly.

 IB Docs (2) Team
IB Docs (2) Team 








