18.2 Calculations involving acids & bases
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 18.2 Calculations involving acids & bases. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
 Learning outcomes
Learning outcomes
 After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- How to express the dissociation constant of a weak acid, Ka and a weak base, Kb.
- Ka × Kb = Kw for a conjugate acid base pair.
- pKa = − log10 Ka and pKb = − log10 Kb.
- Solve problems involving [H+(aq)], [OH–(aq)], pH, pOH, Ka, pKa, Kb and pKb.
- Discuss the relative strengths of acids and bases using values of Ka, pKa, Kb and pKb.
Relationships & vocabulary
Nature of science
By applying the equilibrium law the strengths of acids and bases can be determined and related to their molecular structure thus providing evidence for scientific theories.
International-mindedness
This topic is mathematical in nature. Although chemists around the world speak many different languages, the fact that mathematics is a universal language enables them to communicate more objectively.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 8 & 18: Acids & bases.
Vocabulary
| ionic product of water, Kw | pOH | acid dissociation constant, Ka | base dissociation constant. Kb | pKa and pKb |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
How weak is a weak acid?
The IB does not require you to be able to solve quadratic equations when it comes to acids and bases calculations. This means that you are required to make an assumption that the equilibrium concentration of a weak acid in solution is the same as the concentration of the undissociated acid. How valid is this assumption?
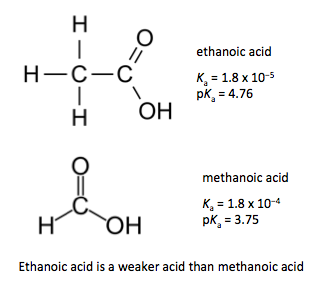
Consider a 0.100 mol dm-3 aqueous solution of ethanoic acid (Ka = 1.8 x 10-5)
CH3COOH(aq) ⇄ CH3COO–(aq) + H+(aq)
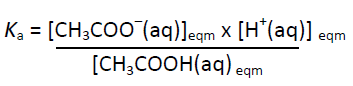
Since water is only very slightly dissociated (Kw = 1.00 x 10-14 at 298 K)
[CH3COO–(aq)] = [H+(aq)] and [CH3COOH(aq)]eqm = (0.100 – [H+(aq)])
[H+(aq)]2 = 1.8 x 10-5 x (0.100 – [H+(aq)])
[H+(aq)]2 – (1.8 x 10-5 x [H+(aq)]) – 1.8 x 10-6 = 0
Solving the quadratic equation gives [H+(aq)] = 1.33 x 10-3 mol dm−3 which gives a pH value of 2.9
If the approximation is made that the equilibrium concentration of the acid is the same as the undissociated acid then
[H+(aq)]2 = 1.8 x 10-5 x 0.100 = 1.8 x 10-6 mol2 dm−6
[H+(aq)] = (1.8 x 10-6)½ = 1.34 x 10-3 mol dm−3 which gives a pH value of 2.9
Clearly the difference is quite small so the approximation is valid.
However consider a weak solution of a stronger acid e.g. 1.00 x 10−3 mol dm−3 dichloroethanoic acid.
CHCl2COOH(aq) ⇄ CHCl2COO–(aq) + H+(aq)
From Section 21 of the data booklet, pKa of dichloroethanoic acid = 1.35; so Ka = 10−1.35 = 4.5 x 10-2
Without the approximation:
[H+(aq)]2 – 4.5 x 10-2[H+(aq)] – 4.5 x 10-5 = 0
which solves to give [H+(aq)] = 9.79 x 10-4 mol dm−3 (pH value of 3.0)
Using the approximation:
[H+(aq)] = (4.5 x 10−2 x 1.00 x 10−3)½ = 6.7 x 10-3 mol dm−3 (pH value of 2.2)
Now there is clearly a large difference and the approximation is not valid. You need to understand that the approximation only really works for relatively concentrated solutions of relatively weak acids. Ostwald’s dilution law is beyond the syllabus but generally the acid should be less than 5% dissociated for the approximation to be valid.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Calculations involving acids & bases.
For short-answer questions see Acid-base calculations questions.
More resources
1. A pdf file on acid base calculations ![]() from Stephen K. Lower at Simon Fraser University goes a little bit further than the IB requires but is a good source of background information.
from Stephen K. Lower at Simon Fraser University goes a little bit further than the IB requires but is a good source of background information.
2. There are many videos (of varying quality) on acid base calculations. Richard Thornley, a teacher at the International School of Genoa, has made some especially for the IB. This shows calculations involving how Kw varies with temperature.
3. Ranking acids and bases in order of strength according to their Ka, Kb, pKa and pKb values also by Richard Thornley.

 IB Docs (2) Team
IB Docs (2) Team 














