20.3 Stereoisomerism
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 20.3 Stereoisomerism. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
 Learning outcomes
Learning outcomes
After studying this sub-topic you should be able to:
 Understand:
Understand:
- There are two sub-classes of stereoisomers - conformational isomers and configurational isomers
- Conformational isomers interconvert by rotation about a σ bond.
- Configurational isomers can only interconvert by breaking and reforming a bond.
- Configurational isomers are further subdivided into E/Z and cis/trans isomers and optical isomers.
- Cis/trans isomers can occur in alkenes or cycloalkanes (or heteroanalogues) and differ in the positions of atoms (or groups) relative to a reference plane.
- E/Z isomerism refers to alkenes of the form R1R2C=CR3R4 (R1 ≠ R2, R3 ≠ R4) where neither R1 nor R2 need to be different to R3 or R4.
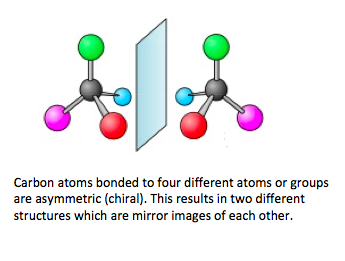
- An asymmetric (joined to four different atoms or groups) carbon atom is called a chiral carbon atom.
- Optically active compounds can rotate the plane of plane-polarized light as it passes through a solution of the compound.
- Optical isomers are known as enantiomers.
- Enantiomers are non-superimposable mirror images of each other.
- Diastereomers are not mirror images of each other.
- A 50:50 mixture of two enantiomers is known as a racemic mixture (or racemate) and is optically inactive.
Apply your knowledge to:
- Construct real or virtual 3-D models of different stereoisomers.
- Explain stereoisomerism in non-cyclic alkenes and in three and four membered cycloalkanes.
- Compare the physical and chemical properties of enantiomers.
- Describe and explain optical isomers in simple organic molecules.
- Use a polarimeter to distinguish between optical isomers.
Relationships & vocabulary
Nature of science
The three-dimensional shape of an organic molecule is fundamental to both its structure and properties. Many of the molecules in the human body are chiral molecules that influence and determine how the body functions. This exemplifies the transdisciplinary nature of stereoisomerism.
International-mindedness
Drugs and medicines produced by pharmaceutical companies around the world have been sold and administered as racemic mixtures instead of as the desired enantiomer with the associated therapeutic
activity? A well-documented example of this is thalidomide.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 10 & 20 : Organic chemistry.
Vocabulary
| conformational isomer | racemic mixture | chiral | E/Z | racemate |
| configurational isomer | CIP (Cahn–Ingold–Prelog) priority | enantiomer | diastereomer | polarimeter |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
The IB distinguishes between conformational isomers and configurational isomers. However as conformational isomers exist in dynamic equilibrium their individual properties cannot easily be studied so this sub-topic is really only concerned with configurational isomers. In teh past these were formally sub-divided into geometric and optical isomers but the use of the term geometric isomers is no longer recommended.
The E/Z system is actually quite easy to apply and depends on what are known as the Cahn-Ingold-Prelog (CIP) rules for determining the priority of the atoms or groups attached to the two carbon atoms of the double bond. Each of these two carbon atoms on either side of the double bond is considered separately. In simple terms the higher the atomic number of the attached atoms to each carbon atom the higher the priority. Consider 1,2-dichloroethene. Chlorine has a higher atomic number than hydrogen so has a higher priority. If the two atoms/groups lie on the same side the isomer is Z and if they lie on opposite sides they are E. In this simple case Z is the cis- form and E is the trans- form.
Cl has a higher priority than H on the left hand side and on the right hand side.
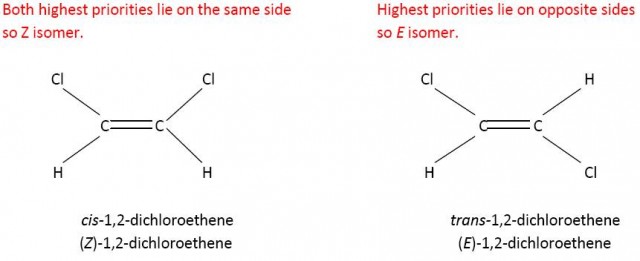
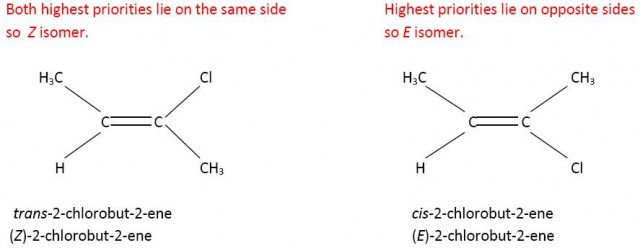
However Z does not always equate to cis-. If you consider 2-chloro-but-2-ene then the carbon atom of the methyl group has priority over the hydrogen atom on one side of the double bond but because chlorine has a higher atomic number than carbon the chlorine atom has priority over the methyl group on the other side of the double bond. Now the cis- isomer is the E isomer and the trans-isomer is the Z isomer.
CH3- has a higher priority than H on the left hand side and Cl has a higher priority than CH3- on the right hand side.
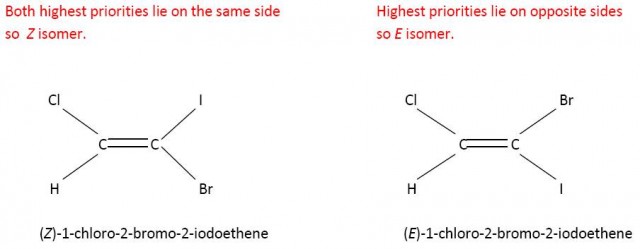
E and Z is more useful than cis- and trans- when the groups are all different. Consider 1-chloro-2-bromo-2-iodoethene. It is not obvious which would be the cis- and trans- forms. However using the Cahn-Ingold-Prelog rules the E and Z forms can easily be determined.
Cl has a higher priority than H on the left hand side and l has a higher priority than Br on the right hand side.
To help you it is worth knowing the origin of Z and E. Both come from German words. Z is from zusammen (together) and E is from entgegen (opposite). However, if your knowledge of German is not so good, one easy way to remember which is which is that E could stand for enemy, and enemies are on opposite sides.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Stereoisomerism.
For short-answer questions see Stereoisomerism questions.
More resources
1. A good video for clarifying the difference between enantiomers and diastereomers (diastereoisomers).
![]() Enantiomers and diastereoisomers
Enantiomers and diastereoisomers
2. E/Z isomerism explained. This video by Prof. Davis does exactly that.
3. The principles of polarimetry exemplified by HGTCChem.

 IB Docs (2) Team
IB Docs (2) Team 






















