Rate expression & reaction mechanism
 16.1 Rate expression & reaction mechanism (4 hours)
16.1 Rate expression & reaction mechanism (4 hours)
Pause for thought
1. I was searching around on the Internet and came across a worksheet with questions on the rate expression.
The first two questions are
1) Write the following for the reaction N2 + 3H2 → 2NH3
- The rate expression for the reaction
- The order of the reaction in each of the reagents
- The overall order of the reaction
2) The rate constant for the reaction HNO3 + NH3 → NH4NO3 is 14.5 L / mol.sec.
If the concentration of nitric acid is 0.050 M and the concentration of ammonia is 0.10 M, what will the rate of this
reaction be?
The answers are then given:
1).
- Rate = k[N2][H2]3
- The reaction is first order in nitrogen and third order in hydrogen.
- The overall order of the reaction is fourth order
2). Rate = k[HNO3][NH3]
Rate = (14.5 L / mol.sec)(0.050 M)(0.10 M)
Rate = 0.073 mol / L. sec
 The question uses M and L /mol. sec whereas we use mol dm-3 and dm-3 mol -1 s-1 and no states are given and k is not in italics but all that is only a question of convention. What is completely wrong is that these questions are unanswerable with the information given. Rate expressions cannot be determined from the stoichiometric equation. They can only be arrived at from experimental data.
The question uses M and L /mol. sec whereas we use mol dm-3 and dm-3 mol -1 s-1 and no states are given and k is not in italics but all that is only a question of convention. What is completely wrong is that these questions are unanswerable with the information given. Rate expressions cannot be determined from the stoichiometric equation. They can only be arrived at from experimental data.
The rate equation for the first question can only be expressed as:
rate = k[N2]x[H2]y
where x and y are the order of the reaction with respect to nitrogen and hydrogen and must be found by experiment.
In fact work by Ertl and others have shown that in the Haber Process (right) where an iron catalyst is used the reaction is complex and thought to proceed by the following steps:
- N2 (g) → N2 (adsorbed)
- N2 (adsorbed) → 2 N (adsorbed)
- H2(g) → H2 (adsorbed)
- H2 (adsorbed) → 2 H (adsorbed)
- N (adsorbed) + 3 H(adsorbed)→ NH3 (adsorbed)
- NH3 (adsorbed) → NH3 (g)
The second step is the slowest step which suggests that [H2] does not even appear in the rate equation.
Another example to illustrate the same point is the reaction of hydrogen with halogens:
H2(g) + X2(g) → 2HX(g)
When X is iodine, the experimentally determined rate expression looks as if it could be deduced from the stoichiometric equation as it is:
rate = k[H2(g)][l2(g)]
but it is very different case when bromine is involved
rate = k[H2(g)][Br2(g)]½ / ([Br2(g)] + k'[HBr(g)]
2. Just a question concerning reaction mechanisms that a student of mine once asked which you might like to reflect upon.
We were looking at nucleophilic substitution reactions and specifically why the hydroxide ion is a better nucleophile than water. During the discussion I confirmed the answer given by most of the students that the hydroxide ion is more electron rich and therefore it will be attracted more strongly to the δ+ carbon atom of the halogenoalkane. Her question was: How can we know that hydroxide ions are better nucleophiles than water in the case of tertiary halogenoalkanes? She argued that when the halogenoalkane is tertiary the first step in the reaction is the dissociation of the halogenoalkane into the halogen ion and the tertiary carbocation. Since this is a first order reaction with the rate only depending upon the concentration of the halogenoalkane the first step is the slow step in the reaction. Therefore the nucleophile will play no part in the overall rate and so there should be no difference in the rate when either water or hydroxide ions are used as the nucleophile.
SN2 mechanism. The better nucleophile should give a faster rate as both the nucleophile and the halogenoalkane are involved in the rate determining step:
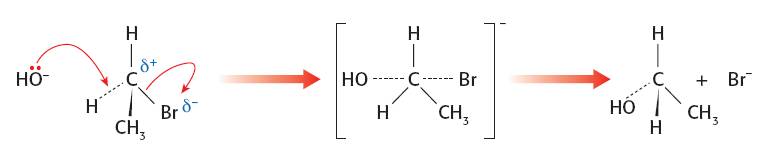
SN1 mechanism. The first step is the slow step with only the halogenoalkane involved:

Since the second step is faster the nature of the nucleophile should make no difference to the overall rate of the reaction so it is impossible to determine which is the better nucleophile in this situation:
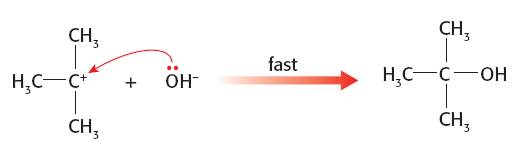
She was a smart student!
Nature of Science
Rather like 6.1 : Collision theory and rates of reaction, this sub-topic also provides a good example of the Principle of Occam’s razor. As theories develop they need to remain as simple as possible while maximizing their powers of explanation. Because the probability of collisions between three reacting species is low it means that stepwise reaction mechanisms are more likely.
Learning outcomesAfter studying this topic students should be able to: Understand
Apply their knowledge to:
| Clarification notesCalculations will only involve orders with whole number values. International-mindednessThe first industrial catalyst to be used was for the Contact process to produce sulfuric acid. It has been stated that for a long time the amount of sulfuric acid produced by a country closely mirrored the country’s economic health. |
Teaching tipsFrom the core it is important to stress the definition of rate of reaction as the units, mol dm-3 s-1 are crucial when it comes to deducing the different units for the rate constants for zero, first, second and third order reactions. Use the example I've given above (or another one) to stress that rate expressions cannot be arrived at just by looking at the stoichiometric equation. They have to be derived from experimental evidence. Use that time old reaction A + B → products to give the general expression rate = k[A]x[B]y to explain what is meant by order of reaction with respect to a particular reactant and the overall order of reaction. Stress the difference between rate constant (small italic k) and equilibrium constant (large italic K). Deducing the order from experimental data almost always for the IB means looking at keeping all the concentrations of the reactants constant and just doubling or tripling one of them selectively to see the effect on the initial rate (see the attached questions). If students understand the concepts behind rate expression they should be able to deduce the shapes of the concentration v time and rate v concentration graphs for zero, first and second order reactions. It is not specified on the syllabus but it is worth talking about half-life, t½, and getting them to realise that this can be used to show whether a reaction is first order by seeing if the half-life on the concentration v time graph is constant. The most important concept for reaction mechanism is that the overall rate of a reaction depends only on the slow step. This is perhaps not immediately obvious to students. I get them to stand in a row and pretend they are on the production line of a factory (e.g. to make cars) with each being given a particular job. As the line rolls past they quickly see that the rate at which cars are produced depends on whoever is slowest, e.g. the one putting the wheels on, and that it doesn't matter how fast the others work the overall rate will not change. This topic relates well to the SN1 and SN2 mechanisms for organic substitution reactions so it is certainly worth making the link (as I have done in 'pause for thought' above). Stress that the order of the reaction and hence the rate equation can only be determined experimentally. Stress too that it is impossible to prove a mechanism. All that you can do is propose a mechanism that is consistent with the rate equation. Mechanisms cannot be deduced just by looking at the chemical equation for the reaction possible mechanisms must be deduced from the rate equation. It is worth explaining that collisions between three particles are very unlikely so all rate determining steps will only involve one or two species although one of the species may be an intermediate. The reaction between hydrogen and nitrogen(II) oxide to produce nitrogen and water is a good example of this. The reaction is first order with respect to hydrogen and second order with respect to nitrogen(II) oxide. This is because the mechanism is thought to be: | Study guide
Page 49 - 50 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MCTest: Rate expression & reaction mechanisms. For short-answer questions on rate expressions which can be set as an assignment for a test, homework or given for self study together with model answers see Rate expression questions. For short-answer questions on reaction mechanisms which can be set as an assignment for a test, homework or given for self study together with model answers see Reaction mechanisms questions. Vocabulary listrate constant, k IM, TOK, 'Utilization' etc.See separate page which covers all of Topics 6 & 16 Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. Deducing the rate expression by looking at initial rates by Richard Thornley, an IB teacher at the International school of Genoa. The video also looks at the units of the rate constant, k, although this is explained more in the next video.
2. Another Richard Thornley video - this time on order or reaction and rate constants.
3. A nice visual demonstration by Richard Thornley showing the importance of the rate determining step.
4. There are a lot of 'lecture' type video clips on YouTube on reaction mechanisms. Here is one by Mark Rosengarten.

 IB Docs (2) Team
IB Docs (2) Team 





























