Nucleophilic substitution
 20.1 Types of organic reactions (7 hours)
20.1 Types of organic reactions (7 hours)
1. Nucleophilic substitution reactions (Estimated 2 hours)
Pause for thought
1. Use of curly arrows
When teaching and describing organic mechanisms involving the use of curly arrows it is much clearer to use 3-D diagrams rather than 2-D diagrams. A curly arrow represents the movement of a pair of electrons from where they start to where they end up. If you only use a 2-D diagram this can cause considerable confusion. Consider the substitution of the bromine atom in bromoethane by the hydroxide nucleophile. Firstly the curly arrow should start from a lone pair on the oxygen atom of the hydroxide ion not from the hydrogen atom - but where should the arrowhead go? Some books show it going to the carbon atom but the pair of electrons ends up forming the bond between the carbon atom and the oxygen atom of the -OH group so the arrowhead should really be going there. In 2-D this is exactly where the electron pair between the carbon atom and the bromine atom is already located. So the diagram will look like this:
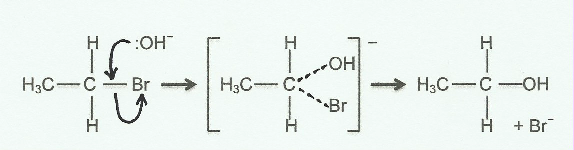
Although 'correct' in 2-D there are two big problems. The first is that the existing electron pair forming the C-Br bond will repel the incoming electrons and the second is that the angle between the C….OH and C....Br bonds in the transition state looks to be 90o or even less. In reality the hydroxide nucleophile will approach the δ+ carbon atom of the polar C—Br bond from the opposite side. This means that the bond angle between the C....OH and the C....Br bonds in the transition state will be 180o. This can easily be shown using a 3-D representation and avoids all the problems caused by a 2-D diagram.
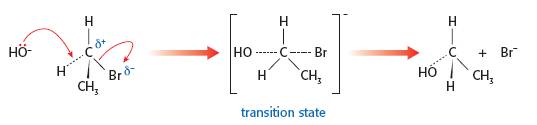
Full details about using curly arrows correctly (and the common errors that students make) can be found on the page 'Curly arrows' in Areas of difficulty. It is worth talking about the work of Paul Walden (1863 - 1957), a Latvian chemist, who provided strong evidence for the SN2 mechanism using secondary halogenoalkanes. If an optically active halogenoalkane is used and the nucleophile does 'attack' from the other side then the product should rotate the plane of plane-polarized light in the opposite direction, which is indeed the case. This is known as Walden inversion and is a good way to bring some TOK into the lesson and also cover this part of the syllabus.
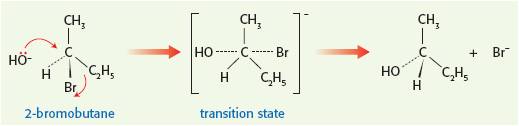
2. How important are electron deficient carbon atoms?
Chemistry is not always as simple and obvious as it would appear to be. When describing the mechanism for nucleophilic substitution it is usual to state that the nucleophile, which contains a non-bonding pair of electrons, is attracted to a carbon atom deficient in electrons in some way. This electron deficient carbon atom may be an actual positive ion as it is in the case of SN1 where the first step is the breaking of the carbon-halogen bond to form an intermediate carbocation. Alternatively it may be the carbon atom in the halogenoalkane which is electron deficient due to the more electronegative halogen atom and hence attracts the nucleophile as in the SN2 mechanism.
This is normally shown by using δ+ and δ– with the nucleophile being attracted to the δ+ on the carbon atom.

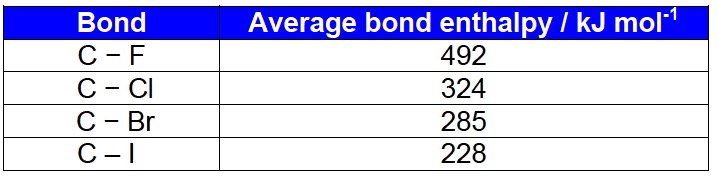
It would seem logical that the stronger the polarity of the carbon-halogen bond the more the nucleophile will be attracted to the δ+ carbon atom. The strength of the polarity will depend on the electronegativity of the halogen compared to the carbon atom. By far the most polar bond is the carbon to fluorine bond.
Difference in electronegativity values for the carbon-halogen bond
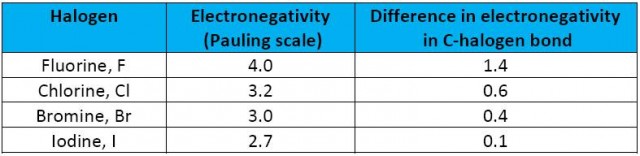
On this logic alone one would expect nucleophiles to react most readily with fluoroalkanes and least readily with iodoalkanes. In fact exactly the reverse is the case. Fluoroalkanes are generally so unreactive that one of their main uses is as fire extinguishers and they are not even included in this sub-topic. From their own experimental work students will be able to see that 1-iodobutane, for example, reacts much faster with nucleophiles than 1-brombutane which in turn reacts much faster than 1-chlorobutane. This suggests that the polarity of the carbon atom is relatively insignificant and that some other factor must be dictating the ease of the reaction.
Even though the 'Application and skills' section uses the words 'Explanation of how the rate depends on the identity of the
halogen (i.e. the leaving group)', there is no mention in the 'Guidance' as to exactly what explanation is required. The syllabus and some books talk about the halogen being a 'leaving group' and it is the ease with which this group leaves that is the important factor, not the polarity of the bond. The ease with which the halogen leaves is, of course related to the strength of the carbon-halogen bond. Iodo- compounds are the most reactive because the carbon-iodine bond is very weak compared to the other carbon-halogen bonds. The fact that the carbon to fluorine bond is so strong accounts for the inertness of fluorocarbons even though they have high polarity.
One other interesting aside concerns the first 'Understandings' statement. It is easy to explain why the hydroxide ion is a better nucleophile than water but for reactions following the SN1 pathway the concentration of the nucleophile does not appear in the rate equation. It could be asked how important is the nature of the nucleophile in the substitution of tertiary halogenoalkanes?
Nature of Science
Organic reactions fall into a number of different categories. By understanding different types of organic reactions and their mechanisms, it is possible to synthesize new compounds with novel properties for use in diverse applications.
Collaboration between scientists on investigating the synthesis of organic compounds using new green chemistry pathways involves ethical and environmental implications.
Learning outcomesAfter studying this sub-topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesStudents should know the difference between homolytic and heterolytic fission and appreciate that SN1 reactions involve heterolytic fission. Emphasise the difference between the use of curly arrows and fish-hooks in reaction mechanisms. The use of partial charges (δ+ and δ-) and 3-D (wedge-dash) representations should be used where appropriate in explaining reaction mechanisms. Although typical conditions and reagents for all reactions should be known (e.g. catalysts, reducing agents, reflux etc.); more precise details, such as specific temperatures, do not need to be included. International-mindednessHow important in the global context is organic chemistry to green and sustainable chemistry? |
Teaching tipsIf you have the luxury of being able to teach Higher Level students on their own rather than together with Standard Level students then it makes sense to cover all of nucleophilic substitution (sub-topics 10.2 and 20.1) in one go. If you have already taught sub-topic 16.1 then it fits in well with rate equations, order of reaction and rate determining steps. Students need to grasp that SN1 depends only on the concentration of the halogenoalkane as the slow step is the breaking of the carbon to halogen bond to form a carbocation. With the SN2 mechanism both the halogenoalkane and the hydroxide ion are involved in the one step mechanism to form a transition state so the rate depends on the concentration of both of them. Impress upon them the difference between a transition state (make sure they include the negative charge outside the square brackets when the hydroxide ion is the nucleophile) and a carbocation intermediate. It can be useful to get them to suggest why primary alcohols go via SN2 (room to get five groups around the central carbon atom) and tertiary halogenoalkanes go via SN1 (stability of the tertiary carbocation). Protic, polar solvents such as water or ethanol, favour SN1 as they support the formation of carbocations and halide ions whereas aprotic, polar solvents, such as propanone, favour the formation of the transition state formed during SN2 reactions. The chemistry guide has been inconsistent in how it has stated this which has caused problems so I have written more about this on the page Factors affecting the rate of nucleophilic substitution reactions in Areas of difficulty. There is no mention of other nucleophiles such as ammonia or cyanide ions on the syllabus. However I would include them and mention that substitution with ammonia can proceed further as the amines produced still contain a non-bonding pair of electrons so can continue to act as nucleophiles. It is also worth pointing out that using the cyanide ion as a nucleophile is a good way to increase the number of carbon atoms in the chain by one. The nitrile produced can then be hydrolysed by dilute hydrochloric acid to a carboxylic acid. Mention too that all nucleophiles act as Lewis bases. Although arguably not on the syllabus you could also discuss why chlorobenzene is very unreactive towards nucleophiles. | Study guide
Page 89 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Nucleophilic substitution. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Nucleophilic substitution questions. Vocabulary list'curly' arrow IM, TOK, Utilization etc.See separate page which covers all of Topics 10 & 20. Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. I've made a small slideshow on Problems with using 'curly arrows'.
2. A rather long (13 minutes) but quite thorough account of SN1 and SN2 and the factors affecting their rate from ChemSurvival. (You will need to explain what tert-butyl means.)
3. SN1 reactions are actually explained (rather than just described) in this good video by Surrey University.
4. A similarly good explanation by Surrey University of the SN2 reaction mechanism.
5. Richard Thornley shows how ammonia can act as a nucleophile and how the product in turn can act as a nucleophile to form primary, secondary, tertiary and quaternary amines.

 IB Docs (2) Team
IB Docs (2) Team 





























