Further aspects of covalent bonding
 14.1 Further aspects of covalent bonding and structure
14.1 Further aspects of covalent bonding and structure
(5 hours)
Pause for thought
Benzene is found in crude oil and is an important industrial chemical, both as a solvent and as a chemical feedstock to make polymers such as polystyrene. It used to be used routinely in schools and universities but since the 1970s it is now illegal to use or store benzene in schools in many countries in the world as it can cause blood disorders and is a known carcinogen.

Benzene (бензол) can still be found in school laboratories in some countries. I took this picture in a school in Kazakhstan.
Even though most students will not deal with benzene directly, it provides one of the classic examples of delocalization. It can be used to illustrate the many different ways in which delocalization is depicted in chemistry. Essentially delocalization in bonding means that a pair of electrons are no longer fixed as a bonding pair between two atoms but are free to move between atoms.
One way of representing delocalisation is through the use of resonance hybrids. These use double-headed arrows and show the extreme forms of the bonding with the true structure lying somewhere between these extremes. For example:

Often a single diagram is used with a dotted or full line to represent the delocalization. For example:
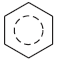 or
or 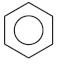
Sometimes the delocalization is shown using the p orbitals and then overlapping them to form delocalized π bonds. For example:
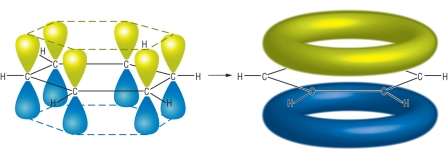
Unless they specify a particular method in a question all of these ways seem acceptable to the IB as descriptions of delocalization. What is important to understand is that in the case of benzene all the C-C bonds are of equal length and of equal energy due to delocalization. Delocalization increases the stability of benzene by about 160 kJ mol-1. This can be used to explain the relative inertness of benzene compared to ethene in undergoing addition reactions as this extra delocalization energy has first to be overcome.
Nature of Science
The way in which bonding theories have been modified over time is an example of Occam's razor as newer theories need to remain as simple as possible whilst maximizing their explanatory power, e.g. including the concept of formal charge.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesCover the linear combination of atomic orbitals to form molecular orbitals in the context of forming σ and π bonds. International-mindednessDiscuss how has the concentration of ozone in the ozone layer has changed over time and what the global community has done to reduce ozone depletion. Discuss whether the global communities efforts to combat ozone depletion are a success or a failure in terms of solving an international environmental concern. |
Teaching tipsThis large topic builds upon sub-topic 3.3 although there is some overlap. For example, resonance structures have already been introduced and also the octet rule and the fact that it is not followed in beryllium and boron compounds. I actually introduce the idea of resonance hybrids first when I am explaining how molecules such as sulfur dioxide or ions such as the carbonate or nitrate ion can be explained using the 'octet rule'. Students should understand that the resonance hybrid forms are used to explain the fact that the S—O bond lengths in SO2 (or SO3) are all equal and lie between a single and a double bond rather than one of them being longer than another which the classic (non-resonance) structure would predict. The use of formal charge is an interesting addition to this syllabus. Like oxidation states it is a man-made concept but it has its uses. Give students plenty of practice at working out the formal charge of simple molecules. You could use it to explain why cyanide ions bond to carbocations using the non-bonding pair of electrons on the carbon atom (rather than the N atom) when they add to carbonyl compounds. You could also give the Data response Example 4 question as that contains much on bonding (e.g. shapes with 5 & 6 electron domains and formal charge). You will need to decide when covering the linear combination of atomic orbitals to form molecular orbitals whether you will talk about anti-bonding orbitals even though they are not mentioned specifically on the syllabus. It does perhaps depend upon the curiosity of your students as they may ask why atomic orbitals combine. In fact bonding is a good example of a modern paradigm and although not on the syllabus it is rather nice to use it to explain why oxygen is paramagnetic even though Lewis theory does not predict this. I introduce the idea of delocalization once I have covered σ and π bonds and hybridization. I use it first to explain benzene and how it represents the two Kekulé resonance hybrid forms. Then I relate it back to other molecules and ions (e.g. NO3–, CO32–, RCOO– and O3) where they have already come across resonance hybrid structures and show that delocalization also works as a good explanation. The concept of delocalization is useful to later explain the strengths of carboxylic acids and extended delocalization (conjugation) to explain why acid-base indicators change colour at different pH. Although the IB does not require it, I also sometimes talk about bond order as it can be a useful concept. So that, for example, the N—O bond order in the nitrate ion is 4/3 (four pairs of electrons spread over three bonds) and the O—O bond order in ozone is 1.5 (three pairs of electrons spread over two bonds). | Study guide
Pages 32 & 33 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Further aspects of covalent bonding. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Further aspects of covalent bonding questions. Vocabulary listsigma bond (σ) IM, TOK, 'Utilization' etc.See separate page which covers all of Topics 4 & 14. Also look at the story of Kekulé on the Emotion and Chemistry page in the TOK section. |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Note that resonance hybrids and incomplete and expanded octets are covered in the slides on Covalent structures (1) and the shapes and polarities of molecules or ions containing five or six electron domains can be found in the slides on Covalent structures (2).
Other resources
1. A good animation of how sigma and pi bonds are formed and how double and triple bonds contain a mixture of sigma and pi bonds by Richard Thornley.
2. A useful video with examples on calculating formal charge also by Richard Thornley.
3. A slide show using pictures put together by Lawrence Kok on Delocalization and formal charge.

 IB Docs (2) Team
IB Docs (2) Team 

























