Covalent bonding

 4.2 Covalent bonding (3 hours)
4.2 Covalent bonding (3 hours)
Pause for thought
The IB uses Pauling’s scale of electronegativity. It was Linus Pauling who first proposed the idea that the bonding electrons pair between two different atoms is not shared equally. If the electrons are in the same outer electron energy level in both atoms then they will tend to be closer to the atom with the greater number of protons in the nucleus resulting in a polar bond. Similarly the closer the outer electron energy level is to the nucleus then the greater the electronegativity. Thus fluorine is the most electronegative element of all. Pauling’s scale was first proposed in 1932 and is a relative scale so has no units.
In 1934 Robert Mulliken (of the ‘oil drop experiment’ fame) proposed[1] that electronegativity could be measured as a quantitative value measured in kJ mol-1. He arrived at the value by taking the arithmetic mean of the absolute values (i.e. ignoring the + or – sign) for the first ionization energy and the electron affinity of an element. Students could use Section 8 of the IB data booklet to work out values based on this relationship. For example:
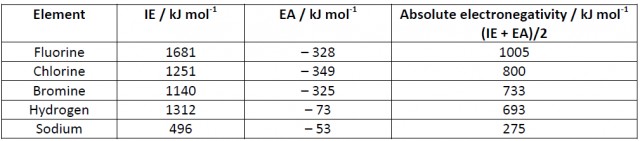
It is an interesting exercise to convert these to the Pauling scale by using the fact that fluorine (1005 kJ mol-1) is equivalent to 4.0 on the Pauling scale. By scaling the other values chlorine (800 kJ mol-1) becomes 800/1005 x 4.0 = 3.2, bromine becomes 733/1005 x 4.0 = 2.9 and by doing similar calculations hydrogen becomes 2.7 and sodium becomes 1.1. These values do compare quite well with Pauling’s scale F (4.0), Cl (3.2), Br (3.0), H (2.2) and Na (0.9). Milliken’s absolute scale is not often used, partly because the electron affinities of some elements are not well-known. Even so perhaps it is worth pointing out to students that there is an absolute scale as well as Pauling’s relative scale. Maybe this could provide some fertile ground for a non-experimental individual scientific investigation?
Nature of Science
Trends and discrepancies - compounds containing only non-metals have different properties than compounds that contain both non-metals and metals.
Natural phenomena can be explained by using theories. For example, Gilbert Lewis introduced a class of compounds which share electrons. Linus Pauling used the idea of electronegativity to explain the unequal sharing of electrons in certain compounds.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesPartial charges, dipoles or vectors can all be used to show bond polarity.Electronegativity values (according to Pauling) are given in Section 8 of the data booklet.
|
Teaching tipsThis is an important topic and one which students need to understand well as it leads on to covalent structures and shapes. The approach I take depends a little on the background of the students because Sub-topic 4.2 does not actually go much further than pre-Diploma level except perhaps for the concept of polarity and electronegativity values. It follows on naturally from ionic bonding and the obvious place to start is with hydrogen. I like to define a single covalent bond as the sharing of a pair of electrons, one from each atom. This holds true for fluorine and chlorine as well as hydrogen. Then I show how atoms can share more than one pair of electrons to produce double and triple bonds (e.g. O2, N2, C2H4 etc.). Stress that double and triple bonds are usually stronger and shorter than single bonds. This prepares students well for the next topic which involves coordinate bonds, the 'octet rule' and Lewis structures. When explaining electronegativity stress the difference between electron affinity (which is a physical quantity measured in kJ mol-1 relating to individual gaseous atoms) and electronegativity which is a relative value and refers to the unequal sharing of bonding electron pairs in a compound. Stress that bonds between two atoms of different elements will always be polar as the electron pair(s) will not be shared equally. However, whether a molecule is polar or not depends on the symmetry of the molecule as the resultant dipole may be zero. | Study guide
Page 26 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Covalent bonding. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Covalent bonding questions. Vocabulary listcovalent bond IM, TOK, 'Utilization' etc.See separate page which covers all of Topic 4 You can also use covalent bonding to illustrate the idea of a paradigm - see the page on A modern paradigm. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A simple but rather good tutorial with a few animations produced by the BBC that might be worth giving to Standard Level students.
2. A good video explaining polar bonds and also polar (e.g. H2O and NH3) and non-polar molecules (e.g. CO2 and CCl4).
Footnotes
- ^ Mulliken, R. S. (1934). "A New Electroaffinity Scale; Together with Data on Valence States and on Valence Ionization Potentials and Electron Affinities". Journal of Chemical Physics 2 (11): 782–793.

 IB Docs (2) Team
IB Docs (2) Team 



















