Classes of compounds & functional groups
Background
The details about homologous series and functional groups covered in sub-topic 10.1 Fundamentals of organic chemistry are quite specific. The homologous series covered, where student should know the general formula (e.g. CnH(2n+1)OH for alcohols) are the alkanes, alkenes, alkynes, ketones, alcohols, aldehydes and carboxylic acids. The syllabus is also clear that students must be able to distinguish between class names (such as alcohol) and the functional group (e.g. hydroxyl). This means students must be able to identify the following classes of organic compounds: alkanes, alkenes, alkynes, halogenoalkanes, alcohols, ethers, aldehydes, ketones, esters, carboxylic acids, amines, amides. They must also be able to identify the functional group contained within these classes, e.g. alkyl, alkenyl, alkynyl, phenyl, hydroxyl, carbonyl, carboxyl, carboxamide, aldehyde, ester, ether, amine and nitrile. This is a significant change from previous syllabuses so not only students but also teachers will need to be careful. This is particularly the case when using past IB questions. In the past, for example, if the question had asked for the functional group present in propan-2-ol the answer would have been alcohol, now it will be hydroxyl. Similarly whereas amide might have been the correct answer to a question in the past now it would be carboxamide. As well as being able to recognise homologous series, classes of compounds and functional groups, the syllabus first published in February 2014 states that students should also be able to name compounds containing only one of the classes of functional groups: alcohols (possibly it should say hydroxyl instead of alcohol here if it is referring to functional groups), ethers, aldehydes, halogenoalkanes, ketones, esters and carboxylic acids for up to six carbon atoms.
The use of carboxamide for amide and carbonyl for a ketone functional group etc. is following what is written in the IUPAC Gold Book. However under ‘alcohols’ the Gold Book states,
“Alcohols - compounds in which a hydroxy group, –OH, is attached to a saturated carbon atom R3COH. The term 'hydroxyl' refers to the radical species, HO..”
This means that the functional group should probably be called hydroxy rather than hydroxyl?
When teaching functional groups and the IUPAC system of naming teachers often simply follow the syllabus and limit themselves to compounds containing up to six carbon atoms. The teaching can get rather repetitive and many of the compounds are not compounds that students can easily relate to. Consequently students often initially tend to muddle the groups up. The structures given in Sections 34, 35 and 37 in the data booklet can be really useful to reinforce identification of both classes of compounds and functional groups. Some examples to ask students are:
Example 1. Alcohols
Find how many compounds there are in Sections 34, 35 and 372 that end in –ol. What functional group do they all have in common? Can you find any other compounds in the tables that also include this group?
Apart from those ending in -ol, i.e. cholesterol, retinol (vitamin A), paracetamol (acetaminophen) and taxol other compounds that contain at least one hydroxyl group are glucose, fructose, ribose deoxyribose, ascorbic acid (vitamin C), vitamin D, quinoidal base (blue), in the flavylium cation (red) (all the three -OH groups shown are joined to an aromatic ring so the class is phenol rather than alcohol), morphine, codeine and zanamivir.
Note that the -OH groups in heme B are both joined to a carbonyl group so they are carboxyl groups not hydroxyl groups.
Example 2. Morphine and heroin
How does the structure of morphine differ from the structure of heroin?
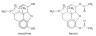
Morphine is a diol (contains two hydroxyl groups) whereas heroin is a diester (contains two ester groups).
Example 3. Aspirin, ibuprofen, penicillin and zanamivir
Aspirin, ibuprofen, penicillin and zanamivir all have one functional group in common – what is it?

They all contain a carboxyl functional group (i.e. they are all carboxylic acids).
Example 4. Vitamin C
Identify the functional groups present in vitamin C

vitamin C
hydroxyl, alkenyl, ester (actually it is a cyclic ester which is not on the syllabus so a student might say carbonyl and ether although this is not correct), alkyl (strictly speaking an -OH group joined to an alkene is called an enol but this is not on the syllabus).
Example 5. Sex hormones
What is the difference between male and female students?
Unfortunately the sex hormones are no longer given in the current data book. However you might like to show your students the structures of testosterone and progesterone to your students and ask them how they are different.

testosterone

progesterone
Both testosterone and progesterone contain a ketone group (hence the ending in –one) but progesterone contains a second ketone group (perhaps it should be called progesterdione?) and testosterone contains an alcohol group (perhaps it should be called testosteronol?). So men contain more alcohol than women!
Example 6. Use your imagination
There are many more ways of using these structures, e.g. is cholesterol a primary, secondary or tertiary alcohol?

secondary

 IB Docs (2) Team
IB Docs (2) Team