Salt formation
Background
The formation, formulas and properties of salts are covered in several topics in both the core and AHL. Formulas and physical properties are covered in sub-topic 4.1 Ionic bonding & structure. Their formation and chemical properties (e.g. products of strong or weak acids or bases) are covered in topic 8. Acids & bases. Electrolysis of molten salts is covered in sub-topic 9.2 Electrochemical cells. At Higher Level the uses of salts in buffer solutions and salt hydrolysis are covered in sub-topic 18.3 pH titration curves.
When you are covering formulas and properties of salts in Topics 4 and 8 the salts of ammonia such as ammonium chloride, NH4+Cl-, will be included.
NH3(aq) + HClaq) → NH4+(aq) + Cl−(aq)
Salts are ionic and tend to be soluble in water and insoluble in non-polar solvents, although there are exceptions. In topic 4 you will also cover hydrogen bonding and will use ammonia as an example of a substance that can exhibit hydrogen bonding due to the fact that hydrogen is bonded to the electronegative nitrogen atom.
It can be interesting to students to pursue this by looking at the structures of some drugs they will be familiar with and use the information to explain some of their properties.
Example
Ask students to use Section 37 to try to identify what the structures of the following well-known but very different drugs all have in common.
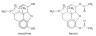

oseltamivir

omeprazole

ranitidine
One answer is that they all contain an amine. To students who have not yet done much organic chemistry an amine can be thought of as an ammonia molecule where one or more of the hydrogen atoms has been substituted by an organic group. Because the N atom of the substituted ammonia still contains a non-bonding pair of electrons and can function as a base, substituted ammonia compounds retain many of the properties of ammonia itself and can from salts.
Ammonia is of course very soluble in water as it can hydrogen bond to water molecules. The substituted ammonia molecules will be much less soluble due the non-polar (or much less polar) organic groups so how can these drugs be made to dissolve in the aqueous medium of blood in order to be carried around the body?
Ionic forms of drugs
Look at the image for heroin (diamorphine) ampoules shown below.
What is actually administered to patients is not pure heroin but its hydrochloride salt. In other words it is just like a substituted form of ammonium chloride. Because it is an ionic salt it is much more soluble in water than heroin itself and hence is more quickly transported around the body. Both morphine and heroin have no hydrogen atoms attached to the N atom so are unable to hydrogen bond to water so they are both almost insoluble in water. However, by converting them into their hydrochloride ionic salts they can be made soluble.
NR3 + HCl(aq) → NR3H+(aq) + Cl−(aq)
Diamorphine hydrochloride is the crystalline form of heroin (diacetylmorphine). It is an extremely strong analgesic and is prescribed to terminally ill cancer patients to speed up the rate it is absorbed by the body. Many other drugs are similarly administered as their hydrochloride salt. There are several other good examples of this on the website. Some are on my blog pages, e.g. Plant food or drug?, Blue light cystoscopy, Applying IB chemistry to a diet pill and Tamoxifen and there is also a newspaper article on Shop until you pop.
Many other drugs such as aspirin and penicillins contain at least one carboxyl functional group (i.e. can be classified as carboxylic acids). These form salts by reacting with bases such as sodium hydroxide. Soluble aspirin is sodium acetylsalicylate, the sodium salt of aspirin. Like all salts it is completely dissociated in water and in the acid environment of the stomach the acetylsalicylate ion accepts a proton to form undissociated aspirin.


 IB Docs (2) Team
IB Docs (2) Team 